3-氯-2-碘甲苯 | 5100-98-1
中文名称
3-氯-2-碘甲苯
中文别名
3-氯-2-碘甲苯,;2-碘-3-氯甲苯
英文名称
1-chloro-2-iodo-3-methylbenzene
英文别名
3-chloro-2-iodotoluene
CAS
5100-98-1
化学式
C7H6ClI
mdl
MFCD00051756
分子量
252.482
InChiKey
FTGLKPMFTLNUBN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:25-27°C
-
沸点:134-136°C 20mm
-
密度:1.806
-
闪点:134-136°C/20mm
-
稳定性/保质期:
在常温常压下保持稳定
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:9
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险类别码:R36/38
-
海关编码:2903999090
-
安全说明:S26,S36/37/39
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 3-Chloro-2-iodotoluene
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H315: Causes skin irritation
H318: Causes serious eye damage
H335: May cause respiratory irritation
H410: Very toxic to aquatic life with long lasting effects
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
P273: Avoid release to the environment
P280: Wear protective gloves/protective clothing/eye protection/face protection
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
Section 3. Composition/information on ingredients.
Ingredient name: 3-Chloro-2-iodotoluene
CAS number: 5100-98-1
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels, refrigerated.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C7H6ClI
Molecular weight: 252.5
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen chloride, hydrogen Iodide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 3-Chloro-2-iodotoluene
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H315: Causes skin irritation
H318: Causes serious eye damage
H335: May cause respiratory irritation
H410: Very toxic to aquatic life with long lasting effects
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
P273: Avoid release to the environment
P280: Wear protective gloves/protective clothing/eye protection/face protection
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
Section 3. Composition/information on ingredients.
Ingredient name: 3-Chloro-2-iodotoluene
CAS number: 5100-98-1
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels, refrigerated.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C7H6ClI
Molecular weight: 252.5
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen chloride, hydrogen Iodide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:3-氯-2-碘甲苯 在 potassium phosphate 、 N-溴代丁二酰亚胺(NBS) 、 [1,1’-bis(diphenylphosphino)ferrocene]dichloropalladium(II) dichloromethane 、 三氟乙酸 、 1,1'-偶氮(氰基环己烷) 作用下, 以 1,4-二氧六环 、 四氯化碳 、 水 、 N,N-二甲基甲酰胺 为溶剂, 反应 42.0h, 生成 2-[2-(6-bromo-2-oxo-1,3-dihydrobenzimidazol-5-yl)-3-chlorophenyl]acetonitrile参考文献:名称:发现和表征对TARP-γ8有选择性的AMPA受体调节剂。摘要:离子型谷氨酸受体的α-氨基-3-羟基-5-甲基-4-异恶唑-丙酸(AMPA)亚型成员介导哺乳动物脑和脊髓内大多数快速突触传递,是治疗干预的有吸引力的靶标。在这里,我们描述了新型AMPA受体调节剂,其需要辅助蛋白CACNG8的存在,也称为跨膜AMPA受体调节蛋白γ8(TARP-γ8)。使用钙通量,放射性配体结合和野生型和突变形式的TARP-γ8的电生理测定,我们证明这些化合物具有新颖的作用机制,与TARP和成孔亚基之间相互作用的部分破坏相一致频道的 分子之一,[5- [2-氯-6-(三氟甲氧基)苯基] -1,3-二氢苯并咪唑-2-酮(JNJ-55511118)具有出色的药代动力学特性,口服后具有较高的受体占有率。该分子对海马内的神经传递具有强烈的剂量依赖性抑制作用,并具有很强的抗惊厥作用。在啮齿动物体内模型中,在高水平的受体占有率下,JNJ-55511118在脑电图上的某些谱带显示出明显的减DOI:10.1124/jpet.115.231712
-
作为产物:描述:参考文献:名称:乙烯基硅基格氏试剂可作为芳烃捕集阱。(芳基烯基)硅烷的新途径。摘要:描述了通过用乙烯基三甲基甲硅烷基格氏试剂亲核捕获芳烃来制备(芳基烯基)硅烷的一般方法。DOI:10.1016/s0040-4039(00)82473-9
文献信息
-
Facile access to sterically hindered aryl ketones via carbonylative cross-coupling: application to the total synthesis of luteolin作者:B. Michael O’Keefe、Nicholas Simmons、Stephen F. MartinDOI:10.1016/j.tet.2011.03.074日期:2011.6achieving the carbonylative cross-coupling of sterically hindered, ortho-disubstituted aryl ketones is reported. The commercially available PEPPSI-IPr catalyst is shown to efficiently promote the carbonylative cross-coupling of hindered ortho-disubstituted aryl iodides to give diaryl ketones; traditional phosphine catalysts are less effective. Carbonylative Suzuki-Miyaura cross-couplings provide a diverse array
-
INDOLE CARBOXAMIDE COMPOUNDS申请人:BRISTOL-MYERS SQUIBB COMPANY公开号:US20160115126A1公开(公告)日:2016-04-28Disclosed are compounds of Formula (I): or a salt thereof, wherein: X is CR 4 or N; R 1 , R 2 , R 3 , R 4 , and A are defined herein. Also disclosed are methods of using such compounds as inhibitors of Bruton's tyrosine kinase (Btk), and pharmaceutical compositions comprising such compounds. These compounds are useful in treating, preventing, or slowing the progression of diseases or disorders in a variety of therapeutic areas, such as autoimmune diseases and vascular disease.
-
[EN] INDANYLOXYDIHYDROBENZOFURANYLACETIC ACIDS<br/>[FR] ACIDES INDANYLOXYDIHYDROBENZOFURANYLACÉTIQUES申请人:BOEHRINGER INGELHEIM INT公开号:WO2012072691A1公开(公告)日:2012-06-07The present invention relates to compounds defined by formula (I) wherein the variables R1, R2, R3, m, and n are defined as in claim 1, possessing valuable pharmacological activity. Particularly, the compounds are activators of the receptor GPR40 and thus are suitable for treatment and prevention of diseases which can be influenced by this receptor, such as metabolic diseases, in particular diabetes type 2.本发明涉及具有式(I)定义的化合物,其中变量R1、R2、R3、m和n如权利要求1中定义,具有宝贵的药理活性。特别是,这些化合物是GPR40受体的激动剂,因此适合用于治疗和预防可以通过这种受体影响的疾病,例如代谢性疾病,特别是2型糖尿病。
-
Rhodium-Catalyzed Atroposelective [2 + 2 + 2] Cycloaddition of <i>Ortho</i>-Substituted Phenyl Diynes with Nitriles: Effect of <i>Ortho</i> Substituents on Regio- and Enantioselectivity作者:Kenichi Kashima、Kota Teraoka、Hidehiro Uekusa、Yu Shibata、Ken TanakaDOI:10.1021/acs.orglett.6b00791日期:2016.5.6Axially chiral 3-(2-halophenyl)pyridines were successfully synthesized in high yields with excellent enantioselectivity by the cationic rhodium(I)/(S)-H8–BINAP complex-catalyzed atroposelective [2 + 2 + 2] cycloaddition of (o-halophenyl)diynes with nitriles. Interestingly, regio- and enantioselectivity highly depend on ortho substituents on the phenyl group of diynes. When the ortho substituents were
-
Carbonylative Cross-Coupling of <i>ortho</i>-Disubstituted Aryl Iodides. Convenient Synthesis of Sterically Hindered Aryl Ketones作者:B. Michael O’Keefe、Nicholas Simmons、Stephen F. MartinDOI:10.1021/ol802202j日期:2008.11.20A mild and general protocol for the carbonylative cross-coupling of sterically hindered ortho-disubstituted aryl iodides is reported. Carbonylative Suzuki-Miyaura couplings of a variety of aryl boronic acids provide an array of substituted biaryl ketones in modest to excellent yield. A carbonylative Negishi coupling that utilizes alkynyl nucleophiles is also described.
表征谱图
-
氢谱1HNMR
-
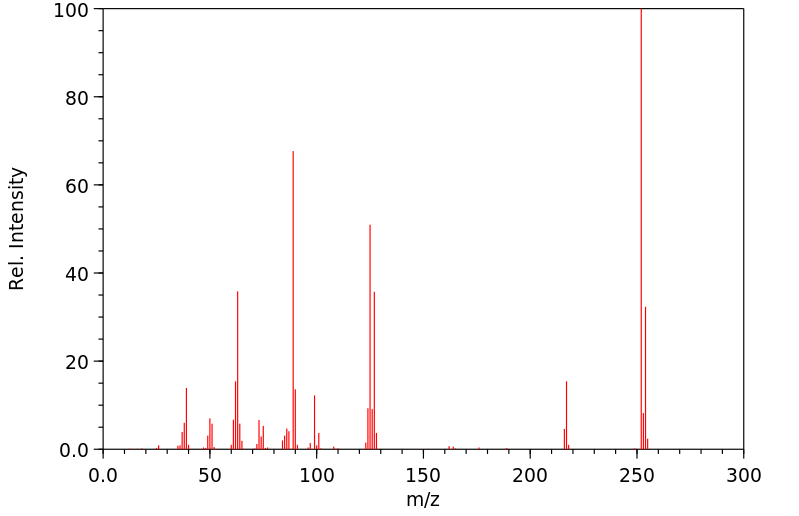
质谱MS
-
碳谱13CNMR
-
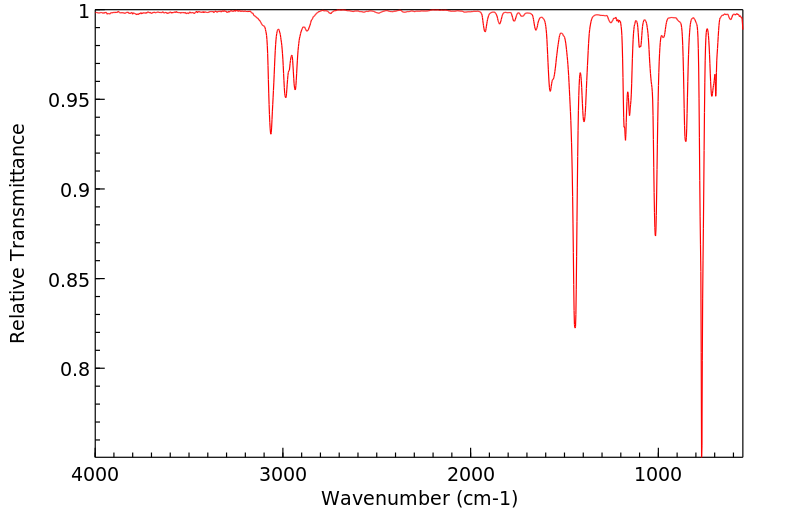
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫