N-(ω-bromoacetoacetyl)-4-methoxyaniline | 20335-27-7
中文名称
——
中文别名
——
英文名称
N-(ω-bromoacetoacetyl)-4-methoxyaniline
英文别名
γ-bromo-p-acetoacetanisidide;ω-bromoacetoacet-para-anisidide;4-bromo-4'-methoxyacetoacetanilide;ω-Bromacetoacet-p-anisidid;N-(4-Brom-3-oxo-butyryl)-4-methoxy-anilin;4-bromo-N-(4-methoxyphenyl)-3-oxobutanamide
CAS
20335-27-7
化学式
C11H12BrNO3
mdl
——
分子量
286.125
InChiKey
URSFPJFWRCLFRU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:16
-
可旋转键数:5
-
环数:1.0
-
sp3杂化的碳原子比例:0.27
-
拓扑面积:55.4
-
氢给体数:1
-
氢受体数:3
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 乙酰基乙酰对甲氧基苯胺 4'-methoxyacetoacetanilide 5437-98-9 C11H13NO3 207.229
反应信息
-
作为反应物:描述:N-(ω-bromoacetoacetyl)-4-methoxyaniline 在 sodium tetrahydroborate 作用下, 以 乙醇 为溶剂, 生成 4-Bromo-3-hydroxy-N-(4-methoxy-phenyl)-butyramide参考文献:名称:Tabei, Katsumi; Ito, Hideharu; Takada, Toyozo, Heterocycles, 1980, vol. 14, # 11, p. 1779 - 1784摘要:DOI:
-
作为产物:描述:参考文献:名称:药物诱导的免疫应答修饰。12.作为新型抗过敏剂的4,5-二氢-4-氧代-2-(取代的氨基)-3-呋喃羧酸及其衍生物。摘要:描述了一系列新颖的4,5-二氢-4-氧代-2-(取代的氨基)-3-呋喃羧酸,盐,酯和酰胺的合成。在大鼠介导的皮肤血管通透性和活性过敏试验中进行测试时,标题化合物表现出中度至强效的抗过敏活性。出现了[2-反式-(4-甲基苯基)环丙基]氨基类似物53作为最有活性的衍生物。因此,当以100 mg / kg的剂量腹膜内给予大鼠时,它可抑制血清素,组胺和缓激肽等介质的作用达100%。在大鼠的主动过敏反应测定中,腹膜内给药后,化合物30以100 mg / kg的剂量将水肿抑制了81%。DOI:10.1021/jm00118a008
文献信息
-
Visible Light-Induced Pericyclic Cascade Reaction for the Synthesis of Quinolinone Derivatives with an Oxabicyclo[4.2.0]octene Skeleton作者:Guangxing Pan、Shaoheng Qin、Dawen Xu、Fritz E. Kühn、Hao GuoDOI:10.1021/acs.orglett.1c00642日期:2021.4.16A photoinduced pericyclic cascade reaction has been developed to afford oxabicyclo[4.2.0]octenes. Mechanistic studies show that this reaction undergoes [2 + 2]-photocycloaddition, base-promoted elimination, retro-4π-electrocyclization, [1,5]-H shift, and 4π-electrocyclization procedures. This reaction features wide substrate scope, good functional group tolerance, and excellent diastereoselectivity已开发出光诱导的环周级联反应以提供氧杂环[4.2.0]辛烯。机理研究表明,该反应经历了[2 + 2]-光环加成,碱促进的消除,逆向4π-电环化,[1,5] -H移位和4π-电环化程序。该反应具有广泛的底物范围,良好的官能团耐受性和出色的非对映选择性。
-
Studies on 2(1H)-quinolinone derivatives as gastric antiulcer active agents. Synthesis and antiulcer activity of the metabolites of 2-(4-chlorobenzoylamino)-3-(2(1H)-quinolinon-4-yl)propionic acid.作者:MINORU UDHIDA、FUJIO TABUSA、MAKOTO KOMATSU、SEIJI MORITA、TOSHIMI KANBE、KAZUYUKI NAKAGAWADOI:10.1248/cpb.34.4821日期:——The metabolites of 2-(4-chlorobenzoylamino)-3-[2(1H)-quinolinon-4-yl]propionic acid (OPC-12759) (1), which has a potent antiulcer activity towards acetic acid-induced gastric ulcer, were synthesized to confirm their structures and to examine their antiulcer activity. The structures of the major metabolites (2-4) in the rat were identified by means of comparisons with the synthetic compounds. The antiulcer activity of the metabolites (2-4) was found to be lower than that of 1.
-
2-Phenyliminothiazolines, their preparation method and anti-rice blast agent containing the same申请人:——公开号:US20030203950A1公开(公告)日:2003-10-302-phenyliminothiazolines of the following formula I, their salts, their preparation method and their use for treating rice blast. The phenyliminothiazolines and their salts of the present invention have low toxicity to environment or living organisms, and exert high activity at low concentration for treating rice blast through a new controlling mechanism, that is by inhibiting pentaketide synthesis and cyclization of the melanin sythesis process in formula I. 1
-
Synthesis of quinine and quinidine using sulfur ylide-mediated asymmetric epoxidation as a key step作者:Muhammad Arshad、M. Alejandro Fernández、Eoghan M. McGarrigle、Varinder K. AggarwalDOI:10.1016/j.tetasy.2010.04.046日期:2010.7The epoxidation of meroquinene aldehyde with a chiral sulfur ylide as the key step in the synthesis of quinine and quinidine is described. The epoxidation reactions proceed under reagent control with high selectivity and good yield. The effect of sulfide and ylide substituents on the stereochemical outcome of the reaction is discussed. (C) 2010 Elsevier Ltd. All rights reserved.
-
Kumari, Ramesh; Dhindsa, Kuldip Singh; Taneja, A. D., Indian Journal of Chemistry, Section A: Inorganic, Physical, Theoretical and Analytical, 1986, vol. 25, # 6, p. 582 - 584作者:Kumari, Ramesh、Dhindsa, Kuldip Singh、Taneja, A. D.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
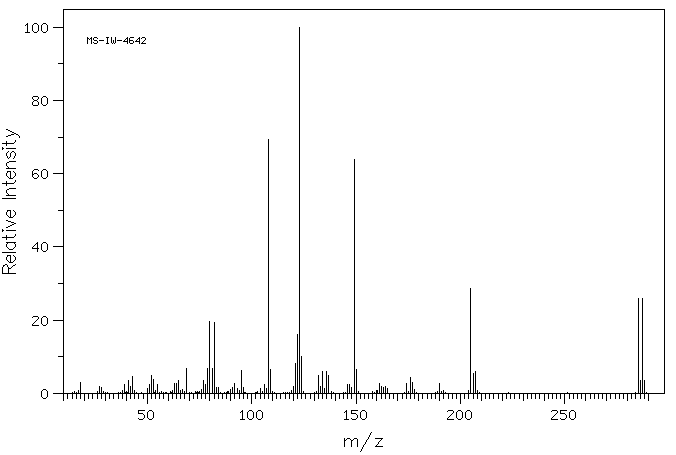
质谱MS
-
碳谱13CNMR
-
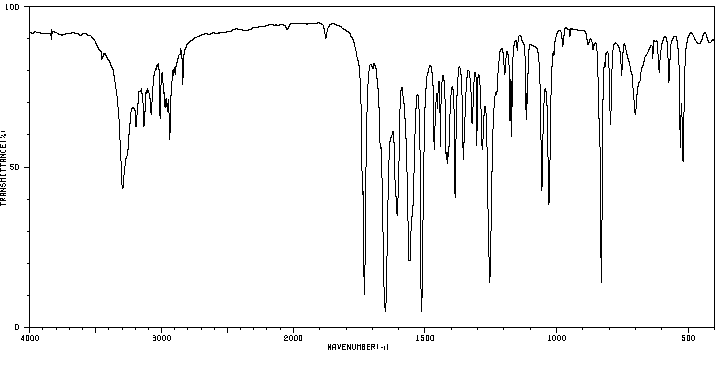
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫