2,3-二氯丙氧基苯 | 39736-21-5
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:287.8±20.0 °C(Predicted)
-
密度:1.209±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.1
-
重原子数:12
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.33
-
拓扑面积:9.2
-
氢给体数:0
-
氢受体数:1
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-氯-3-苯氧基-2-丙醇 (RS)-1-chloro-3-phenyloxy-2-propanol 4769-73-7 C9H11ClO2 186.638 烯丙基苯基醚 allyl phenyl ether 1746-13-0 C9H10O 134.178
反应信息
-
作为反应物:描述:参考文献:名称:CH247179摘要:公开号:
-
作为产物:参考文献:名称:通过 LMCT 激发 CuCl2 可见光诱导烯烃邻位二氯化摘要:摘要这项工作展示了基于 CuCl 均裂的烯烃光氧化还原邻位二氯化反应2响应可见光照射。这种催化通过配体到金属的电荷转移过程进行,并为使用廉价的低分子量氯源合成 1,2-二氯化合物提供了令人兴奋的机会。这种新工艺表现出广泛的底物范围、优异的官能团耐受性、极其温和的条件并且不需要外部配体。机理研究表明,氯原子自由基的快速形成是该合成过程中 C−Cl 键易于形成的原因。DOI:10.1002/ange.202010801
文献信息
-
New applications of tungsten hexachloride (WCl6) in organic synthesis. Halo-de-hydroxylation and dihalo-de-oxo-bisubstitution reactions作者:Habib. Firouzabadi、Farhad. ShirinyDOI:10.1016/0040-4020(96)00905-2日期:1996.11tungsten hexachloride (WCl6) has been used for the halo-de-hydroxylation and dihalo-de-oxo-bisubstitution reactions of benzylic alcohols, benzaldehydes, acyloins, and epoxides to their chlorides, gem-dichlorides, vic- trichlorides, and vic- dichlorides respectively.
-
Trihaloisocyanuric Acid/Triphenylphosphine: An Efficient System for Regioselective Conversion of Epoxides into Vicinal Halohydrins and Vicinal Dihalides under Mild Conditions作者:Marcio de Mattos、Vitor de AndradeDOI:10.1055/s-0035-1560408日期:——vicinal chloro-/bromohydrins and vicinal dihalides by reaction with the system trihaloisocyanuric acid/triphenylphosphine in acetonitrile under mild and neutral conditions. The reactions proceed smoothly in high yield at room temperature and at reflux, respectively, over a short time. A new synthetic method has been developed for the regioselective conversion of epoxides to vicinal chloro-/bromohydrins
-
Rasta resin–triphenylphosphine oxides and their use as recyclable heterogeneous reagent precursors in halogenation reactions作者:Xuanshu Xia、Patrick H ToyDOI:10.3762/bjoc.10.143日期:——
Heterogeneous polymer-supported triphenylphosphine oxides based on the rasta resin architecture have been synthesized, and applied as reagent precursors in a wide range of halogenation reactions. The rasta resin–triphenylphosphine oxides were reacted with either oxalyl chloride or oxalyl bromide to form the corresponding halophosphonium salts, and these in turn were reacted with alcohols, aldehydes, aziridines and epoxides to form halogenated products in high yields after simple purification. The polymer-supported triphenylphosphine oxides formed as a byproduct during these reactions could be recovered and reused numerous times with no appreciable decrease in reactivity.
-
Regioselective synthesis of <i>vic</i>-halo alcohols and symmetrical or unsymmetrical <i>vic</i>-dihalides from epoxides using triphenylphosphine <i>N</i>-halo imides作者:Nasser Iranpoor、Habib Firouzabadi、Roya Azadi、Farzaneh EbrahimzadehDOI:10.1139/v05-261日期:2006.1.1
A simple, novel, and highly regioselective cleavage of epoxides into vicinal halo alcohols and symmetrical or unsymmetrical dihalides is described using different stoichiometries of triphenylphosphine (PPh3) and N-halo succinimide (NXS) or N-halo saccharine (NXSac).Key words: N-halo succinimide (NXS), N-halo saccharine (NXSac), triphenylphosphine (PPh3), epoxide, vic-halo alcohol, symmetrical dihalide, unsymmetrical dihalide.
-
Single-Isomer Iodochlorination of Alkynes and Chlorination of Alkenes Using Tetrabutylammonium Iodide and Dichloroethane作者:Michael L. Ho、Alison B. Flynn、William W. OgilvieDOI:10.1021/jo062188w日期:2007.2.1The efficient formation of single-isomer, differentially halogenated alkenes and alkanes is described. These structures were generated by treatment of the appropriate alkyne or alkene with tetrabutylammonium iodide in refluxing dichloroethane. This process is highly selective as evidenced by control experiments using ICl. Treatment of the same alkenes and alkynes directly with iodine monochloride resulted
表征谱图
-
氢谱1HNMR
-
质谱MS
-
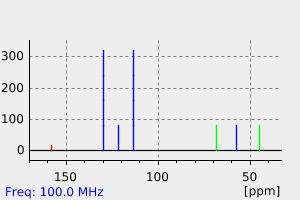
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息