1,4,5,8,9,10-六氢蒽 | 5910-28-1
中文名称
1,4,5,8,9,10-六氢蒽
中文别名
——
英文名称
1,4,5,8,9,10-hexahydroanthracene
英文别名
1,4,5,8,9,10-Hexahydroanthracen
CAS
5910-28-1
化学式
C14H16
mdl
MFCD00001186
分子量
184.281
InChiKey
LNRAWXJRDXDHJN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:147-150°C
-
沸点:156°C
-
密度:0.9799 (estimate)
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:14
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.428
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
包装等级:I; II; III
SDS
| Name: | 1 4 5 8 9 10-Hexahydroanthracene 99% Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 5910-28-1 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 5910-28-1 | 1,4,5,8,9,10-hexahydroanthracene | 99.0 | 227-621-1 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Never give anything by mouth to an unconscious person. Get medical aid immediately.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Antidote: None reported.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Keep container closed when not in use. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 5910-28-1: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 147.00 - 150.00 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: N/A
Explosion Limits, upper: N/A
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C14H16
Molecular Weight: 184.28
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, strong oxidants.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 5910-28-1 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1,4,5,8,9,10-hexahydroanthracene - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Other No information available.
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 5910-28-1: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 5910-28-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 5910-28-1 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1,2,3,4,5,6,7,8,9,10-decahydroanthracene 3485-60-7 C14H20 188.313
反应信息
-
作为反应物:描述:参考文献:名称:Mashraqui, Sabir; Keehn, Philip, Synthetic Communications, 1982, vol. 12, # 8, p. 637 - 646摘要:DOI:
-
作为产物:描述:参考文献:名称:Vogel,E. et al., Angewandte Chemie, 1966, vol. 78, p. 642 - 643摘要:DOI:
文献信息
-
Organometallic Ruthenium(II) Diamine Anticancer Complexes: Arene-Nucleobase Stacking and Stereospecific Hydrogen-Bonding in Guanine Adducts作者:Haimei Chen、John A. Parkinson、Simon Parsons、Robert A. Coxall、Robert O. Gould、Peter J. SadlerDOI:10.1021/ja017482e日期:2002.3.1[(eta(6)-DHA)Ru(II)(en)Cl][PF(6)] (3, DHA = 9,10-dihydroanthracene) with guanine derivatives, in the solid state by X-ray crystallography, and in solution using 2D [(1)H,(1)H] NOESY and [(1)H,(15)N] HSQC NMR methods. Strong pi-pi arene-nucleobase stacking is present in the crystal structures of [(eta(6)-C(14)H(14))Ru(en)(9EtG-N7)][PF(6)](2).(MeOH) (6) and [(eta(6)-C(14)H(12))Ru(en)(9EtG-N7)][PF(6)](2)[(eta(6)-arene)Ru(II)(en)Cl][PF(6)](en = 乙二胺)类型的有机金属钌 (II) 芳烃抗癌配合物特异性靶向 DNA 寡聚体的鸟嘌呤碱基并形成单功能加合物 (Morris, R., et al. J. Med. Chem. 2001)。我们已经确定了“钢琴凳”复合物的单功能加合物的结构 [(eta(6)-Bip)Ru(II)(en)Cl][PF(6)] (1, Bip = 联苯), [( eta(6)-THA)Ru(II)(en)Cl][PF(6)] (2, THA = 5,8,9,10-四氢蒽),和 [(eta(6)-DHA)Ru( II)(en)Cl][PF(6)] (3, DHA = 9,10-dihydroanthracene) 与鸟嘌呤衍生物,通过 X 射线晶体学呈固态,并在溶液中使用 2D [(1)H,( 1)H] NOESY 和 [(1)H,(15)N]
-
Electroreduction in aqueous media, saturation of polycyclic aromatics作者:Essie Kariv-Miller、Ryszard I. PacutDOI:10.1016/s0040-4020(01)90597-6日期:1986.1constant-current and the products were isolated and identified. Anthracene formed initially 9,10-dihydroanthracene which reduced with additional charge transfer to 1,4,5,8,9,10-hexahydroanthracene in high yield. To obtain more saturated anthracene derivatives, a process was developed which involves a “one pot” reduction-isomerization-reduction sequence. Anthracene was electrolyzed first to completion, the product研究了蒽和菲的制备型阴极还原,旨在获得水溶液中的桦木型产物。根据先前的报道,使用汞池阴极和四丁基铵电解质进行了还原。发现最佳条件包括氢氧化四丁铵作为电解质和水作为唯一溶剂。使用恒流在简单的电池中进行反应,并分离和鉴定产物。蒽最初形成9,10-二氢蒽,然后通过额外的电荷转移将其还原为高产率的1,4,5,8,9,10-六氢蒽。为了获得更多的饱和蒽衍生物,开发了一种涉及“一锅”还原-异构化-还原序列的方法。蒽首先被电解完成 产物通过在电解液(在池中但没有电荷转移)中的回流进行异构化,然后再次进行电解。使用该方法以显着的产率形成了1,2,3,4,5,6,7,8-八氢蒽和1,2,3,4,5,6,7,8,9,10-十氢蒽。菲的还原导致高饱和衍生物的混合物,而无需异构化步骤。主要产物随二氢菲()合成八氢菲()和十氢菲()。通常显示出在水溶液中多环芳族化合物的阴极加氢是可能的。产物类似于通过金属氨或胺还原
-
Crystal Structures of Conformationally Locked Cyclitols: An Analysis of Hydrogen-Bonded Architectures and their Implications in Crystal Engineering作者:Goverdhan Mehta、Saikat Sen、Senaiar S. RameshDOI:10.1002/ejoc.200600610日期:2007.1qualitative study has been carried out on selected polycyclitols to evaluate the potential of conformational locking of hydroxy groups in lending predictability to the O–H···O hydrogen-bonding network observed in the crystal structures of such compounds. The polycyclitols employed in this study are conformationally locked with all the hydroxy groups destined to be axial owing to the trans ring fusion(s) in
-
一种电子盐反应液和不饱和芳香烃类化合物的还原方法
-
A safe and convenient new procedure for reducing aromatic compounds to birch-type products作者:Robert A. Benkeser、James A. Laugal、Angela RappaDOI:10.1016/s0040-4039(01)81168-0日期:1984.1Aromatic compounds can be reduced by a calcium-amine-t-butyl alcohol system to products which are identical to those obtained by a Birch reduction of the same substrates.
表征谱图
-
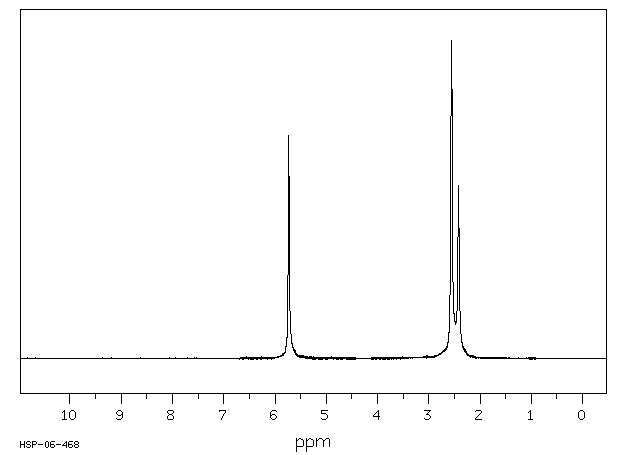
氢谱1HNMR
-
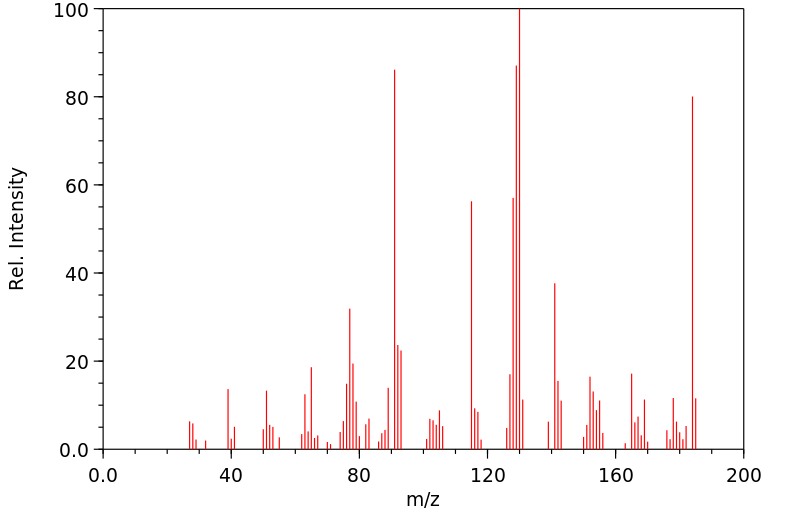
质谱MS
-
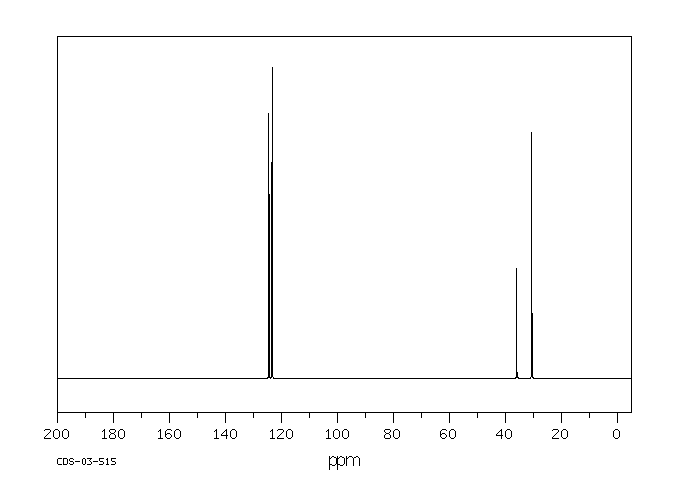
碳谱13CNMR
-
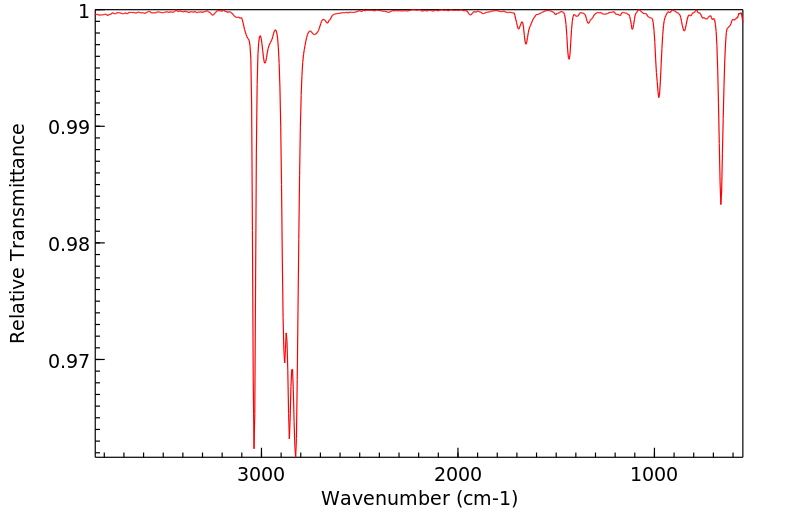
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
齐斯托醌
黄决明素
马普替林相关物质D
马普替林杂质E(N-甲基马普替林)
马普替林杂质D
马普替林D3
马普替林
颜料黄199
颜料黄147
颜料黄123
颜料黄108
颜料红89
颜料红85
颜料红251
颜料红177
颜料紫27
顺式-1-(9-蒽基)-2-硝基乙烯
阿美蒽醌
阳离子蓝FGL
阳离子蓝3RL
长蠕孢素
镁蒽四氢呋喃络合物
镁蒽
锈色洋地黄醌醇
锂钠2-[[4-[[3-[(4-氨基-9,10-二氧代-3-磺基-1-蒽基)氨基]-2,2-二甲基-丙基]氨基]-6-氯-1,3,5-三嗪-2-基]氨基]苯-1,4-二磺酸酯
锂胭脂红
链蠕孢素
铷离子载体I
铝洋红
铂(2+)二氯化1-({2-[(2-氨基乙基)氨基]乙基}氨基)蒽-9,10-二酮(1:1)
钾6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠alpha-(丙烯酰氨基)-[4-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]苯氧基]甲苯磺酸盐
钠[[3-[[4-(环己基氨基)-9,10-二氢-9,10-二氧代-1-蒽基]氨基]-1-氧代丙基]氨基]苯磺酸盐
钠[3-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]丁基]苯磺酸盐
钠6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠4-({4-[乙酰基(乙基)氨基]苯基}氨基)-1-氨基-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠2-[(4-氨基-9,10-二氧代-3-磺基-9,10-二氢-1-蒽基)氨基]-4-{[2-(磺基氧基)乙基]磺酰基}苯甲酸酯
钠1-氨基-9,10-二氢-4-[[4-(1,1-二甲基乙基)-2-甲基苯基]氨基]-9,10-二氧代蒽-2-磺酸盐
钠1-氨基-4-[(3-{[(4-甲基苯基)磺酰基]氨基}苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-[(3,4-二甲基苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-(1,3-苯并噻唑-2-基硫基)-9,10-二氧代蒽-2-磺酸盐
醌茜隐色体
醌茜素
酸性蓝P-RLS
酸性蓝41
酸性蓝27
酸性蓝127:1
酸性紫48
酸性紫43
酸性兰62