5-Chlor-tropolon | 3084-17-1
中文名称
——
中文别名
——
英文名称
5-Chlor-tropolon
英文别名
2,4,6-Cycloheptatrien-1-one, 5-chloro-2-hydroxy-;5-chloro-2-hydroxycyclohepta-2,4,6-trien-1-one
CAS
3084-17-1
化学式
C7H5ClO2
mdl
——
分子量
156.569
InChiKey
QBBVEYLSVFZUQA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:10
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 环庚三烯酚酮 2-hydroxy-2,4,6-cycloheptatrien-1-one 533-75-5 C7H6O2 122.123 2,5-二羟基-2,4,6-环庚并-三烯-1-酮 4,5-dihydroxycyclohepta-2,4,6-trien-1-one 15852-34-3 C7H6O3 138.123 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 5-Chlor-2-methoxytropon 13187-38-7 C8H7ClO2 170.595
反应信息
-
作为反应物:描述:参考文献:名称:The Reaction of Tropolones with Phosphorus Oxychloride. The Rearrangement Reaction of 5-Arylazo-2-chlorotropones to 2-Aryl-4, 5-dichloroindazoles摘要:倍氯酮与磷氧氯化物的反应生成了2-氯倍酮。同样,5-氰基、5-硝基和5-氯倍氯酮也分别获得了相应的2-氯倍酮衍生物,产率适中。3-氰基倍氯酮生成了2-氯-7-氰基倍酮,而3-苯基倍氯酮则仅获得了2-氯-3-苯基苯甲醛。当3-溴倍氯酮和5-溴倍氯酮以类似方式处理时,分别得到2,7-二氯和2,5-二氯倍酮,伴随简单的卤素交换。进一步用磷氧氯化物处理2,5-二氯倍酮,得到2,5-二氯和3,4-二氯苯甲醛。5-p-苯基偶氮、5-苯基偶氮和5-p-硝基苯基偶氮倍酮与磷氧氯化物的反应生成了相应的5-芳基偶氮-2-氯倍酮,经过进一步处理后与过量的试剂反应,得到2-芳基-4,5-二氯吲唑。DOI:10.1246/bcsj.41.424
-
作为产物:描述:参考文献:名称:Improving quality of anesthesia care: opportunities for the new decade摘要:DOI:10.1007/bf03019807
文献信息
-
Biomimetic Formation of 2-Tropolones by Dioxygenase-Catalysed Ring Expansion of Substituted 2,4-Cyclohexadienones作者:Meite Xin、Timothy D. H. BuggDOI:10.1002/cbic.200900631日期:2010.1.25sixes and sevens: We have demonstrated experimentally the proposed ring expansion in the biosynthesis of substituted 2‐tropolones. Treatment of four cyclohexa‐2,4‐dienones with the non‐haem iron(II)‐dependent extradiol catechol dioxygenase MhpB from E. coli resulted in the formation of 2‐tropolones through a pinacol‐type rearrangement. This ring expansion could also be effected nonenzymatically by treatment
-
A versatile new strategy for the synthesis of tropolones作者:Martin G. Banwell、René OnrustDOI:10.1016/s0040-4039(00)88954-6日期:1985.1Swern-type oxidation of various 7-halogenobicyclo[4.1.0]heptane-2,3- or -3,4-diols affords the corresponding bicyclic diketones which undergo in situ ring expansion and loss of hydrogen halide to give α-tropolones in high yield. The quantitative conversion of the isolable 1,4-diketone 26 into the γ-tropolone acetate 27 has been achieved.各种7-卤代双环[4.1.0]庚烷2,3-或-3,4-二醇的Swern型氧化可提供相应的双环二酮,该二环二酮在原位发生环扩环并损失卤化氢,从而在高浓度下得到α-对苯二酚屈服。已经实现了将可分离的1,4-二酮26定量转化为乙酸γ-托酚酮27。
-
4,9-Dihydro-4,9-dioxo-1H-cyclohepta[b]pyridine derivatives申请人:Ayerst, McKenna & Harrison, Inc.公开号:US04381304A1公开(公告)日:1983-04-26Aldose reductase inhibitors of the formula ##STR1## in which R.sup.1 is COOH and R.sup.2 is hydrogen, 8-halo or 6-hydroxy, or R.sup.1 is CON(R.sup.3)-CH.sub.2 COOH wherein R.sup.3 is lower alkyl and R.sup.2 is hydrogen or 8-halo are useful for treating diabetic complications.
-
Sato, Nippon Kagaku Zasshi, 1959, vol. 80, p. 1167,1168作者:SatoDOI:——日期:——
-
[EN] TROPOLONE DERIVATIVES AND TAUTOMERS THEREOF FOR IRON REGULATION IN ANIMALS<br/>[FR] DÉRIVÉS DE TROPOLONE ET LEURS TAUTOMÈRES POUR LA RÉGULATION DU FER CHEZ LES ANIMAUX申请人:AMBYS MEDICINES INC公开号:WO2021076938A8公开(公告)日:2022-04-28
表征谱图
-
氢谱1HNMR
-
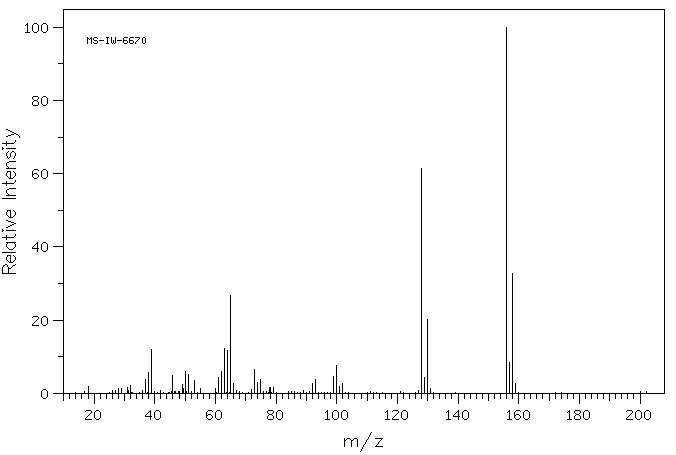
质谱MS
-
碳谱13CNMR
-
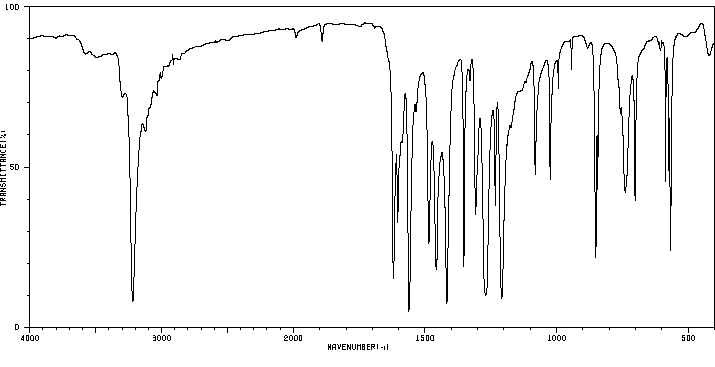
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
脱羰秋水仙碱
红陪酚四甲基醚
红倍酚
秋水仙碱甲硫代磺酸盐
秋水仙碱
硫代秋水仙碱
甲基丙烯酸7-氧代-4-(苯基偶氮)-1,3,5-环庚三烯-1-基酯
甲基6-肼基-7-氧代-1,3,5-环庚三烯-1-羧酸酯
环庚三烯酮
环庚三烯酚酮
氨甲酸,(1-乙基戊基)-,甲基酯(9CI)
桧木醇
异秋水仙胺
尼楚酮
对二硫辛酸
双环[4.4.1]十一碳-1(10),2,4,6,8-五烯-11-酮
双环[4.1.0]庚-1,3,5-三烯-7-酮
去乙酰氨基秋水仙碱
原秋水仙碱
十四烷酸,4-(十八烷氧基)-7-羰基-1,3,5-环庚三烯-1-基酯
乙基[(7S)-1,2,3,10-四甲氧基-9-氧代-5,6,7,9-四氢苯并[a]庚搭烯-7-基]氨基甲酸酯
三甲基秋水仙素酸
三甲基秋水仙素酸
三(2-羟基-2,4,6-环庚三烯-1-酮)-铟
α-(异丙基)-γ,γ-二甲基环己丙醇
beta-斧松素
[(7S)-7-乙酰氨基-1,3-二甲氧基-10-甲硫基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-2-基]2-氯乙酸酯
[(7S)-7-乙酰氨基-1,2-二甲氧基-10-甲硫基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-3-基]2-氯乙酸酯
N-(2-巯基乙基)秋水仙胺
N-脱乙酰基3-去甲基硫代秋水仙碱
N-脱乙酰基,1,2,3,10-脱甲基秋水仙碱
N-甲酰脱乙酰秋水仙碱
N-甲酰基秋水仙胺
N-甲基-秋水仙碱
N-三氟乙酰基-N-甲基-去乙酰基秋水仙碱
N-[(S)-5,6,7,9-四氢-1,2,3,10-四甲氧基-9-氧代苯并[a]庚搭烯-7-基]-2,2,2-三氟乙酰胺
N-[(7S)-4-(羟基甲基)-1,2,3,10-四甲氧基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基]乙酰胺
N-[(7S)-10-(丁基氨基)-5,6,7,9-四氢-1,2,3-三甲氧基-9-氧代苯并[a]庚搭烯-7-基]-乙酰胺
N-[(7S)-1,2,3-三甲氧基-9-氧代-10-(苯基甲硫基)-6,7-二氢-5H-苯并[d]庚搭烯-7-基]乙酰胺
N-[(7S)-1,2,3-三甲氧基-9-氧代-10-(苯基甲基氨基)-6,7-二氢-5H-苯并[d]庚搭烯-7-基]乙酰胺
N-[(7S)-1,2,3,10-四甲氧基-9-氧代-5,6,7,9-四氢苯并[a]庚搭烯-7-基]丙酰胺
N-[(7R)-1,2,3,10-四甲氧基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基]乙酰胺
N-(乙氧基乙酰基)去乙酰基硫代秋水仙碱
N-(5,6,7,9-四氢-1,2,3-三甲氧基-10-甲硫基-9-氧代苯并[a]庚搭烯-7-基)氨基甲酸乙酯
N-(4-甲酰基-1,2,3,10-四甲氧基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基)乙酰胺
N-(10-二甲基氨基-1,2,3-三甲氧基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基)乙酰胺
N-(1,2,3,9-四甲氧基-10-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基)乙酰胺
N-(1,2,3,10-四甲氧基-9-氧代-5,6,7,9-四氢苯并[a]庚搭烯-7-基)乙酰胺
9H-三苯并[A,C,E][7]环轮烯-9-酮
8H-环庚三烯并[c]异噻唑-8-酮,3-甲基-