1,3-二乙基金刚烷 | 25074-51-5
中文名称
1,3-二乙基金刚烷
中文别名
——
英文名称
1,3-diethyladamantane
英文别名
——
CAS
25074-51-5
化学式
C14H24
mdl
——
分子量
192.345
InChiKey
XHQDEOWLMBIVBU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:100-102 °C(Press: 10 Torr)
-
密度:0.942±0.06 g/cm3(Predicted)
-
保留指数:1377;1387;1377;1387
计算性质
-
辛醇/水分配系数(LogP):5.7
-
重原子数:14
-
可旋转键数:2
-
环数:4.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 金刚烷 adamantane 281-23-2 C10H16 136.237 1,3-二羟乙基金刚烷 1,3-di(2-hydroxyethyl)adamantane 80121-65-9 C14H24O2 224.343 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1,3,5-Triethyl-adamantane 25074-54-8 C16H28 220.398
反应信息
-
作为反应物:描述:参考文献:名称:Structure-anti-Parkinson activity relationships in the aminoadamantanes. Influence of bridgehead substitution摘要:A limited series of bridgehead alkyl-, dialkyl-, and trialkyl-substituted amantadines was synthesized and tested for potential anti-Parkinson activity as dopamine (DA) agonists. The compounds were evaluated using a battery of three murine bioassays, including stimulation of locomotor activity, induction of circling in animals with unilateral striatal lesions, and reversal of reserpine/alpha-methyltyrosine induced akinesia. Apparent mechanistic differences were seen between the methyl-substituted series and the ethyl-substituted series. While activities in both series increase with increasing liphophilicity, the methyl series (1b--d), as well as amantadine itself (1a), exhibit only indirect DA agonist activity, as evidenced by ipsilateral rotation in the circling model and no significant difference from control in reversal of akinesia. The ethyl series (1e,f) exhibits weak but reproducible direct DA agonist activity, as shown by contralateral rotation in the circling assay for 1e and reversal of akinesia by 1e and 1f. The 3-n-propyl derivative (1g) was devoid of any DA agonist activity.DOI:10.1021/jm00343a010
-
作为产物:描述:参考文献:名称:Structure-anti-Parkinson activity relationships in the aminoadamantanes. Influence of bridgehead substitution摘要:A limited series of bridgehead alkyl-, dialkyl-, and trialkyl-substituted amantadines was synthesized and tested for potential anti-Parkinson activity as dopamine (DA) agonists. The compounds were evaluated using a battery of three murine bioassays, including stimulation of locomotor activity, induction of circling in animals with unilateral striatal lesions, and reversal of reserpine/alpha-methyltyrosine induced akinesia. Apparent mechanistic differences were seen between the methyl-substituted series and the ethyl-substituted series. While activities in both series increase with increasing liphophilicity, the methyl series (1b--d), as well as amantadine itself (1a), exhibit only indirect DA agonist activity, as evidenced by ipsilateral rotation in the circling model and no significant difference from control in reversal of akinesia. The ethyl series (1e,f) exhibits weak but reproducible direct DA agonist activity, as shown by contralateral rotation in the circling assay for 1e and reversal of akinesia by 1e and 1f. The 3-n-propyl derivative (1g) was devoid of any DA agonist activity.DOI:10.1021/jm00343a010
文献信息
-
一种新的金刚乙胺类似物及其合成方法
-
One pot synthesis of bridgehead amino alcohols from diamantoid hydrocarbons作者:Elena A. Ivleva、Maria S. Zaborskaya、Vadim A. Shiryaev、Yuri N. KlimochkinDOI:10.1080/00397911.2023.2177173日期:2023.3.19Abstract An efficient approach for the synthesis of amino alcohols directly from cage hydrocarbons has been developed. Multigram scalability, good yields and one-pot procedure, that utilize readily available reagents along with synthetic convenience showed its usefulness. The proposed one-pot method includes sequential reactions of cage hydrocarbon with nitric acid, further amination of intermediate
-
One-pot amination of cage hydrocarbons作者:M. V. Leonova、M. Yu. Skomorokhov、I. K. Moiseev、Yu. N. KlimochkinDOI:10.1134/s1070428015120064日期:2015.12A one-pot procedure has been proposed for the synthesis of amines directly from cage hydrocarbons. A number of cage amines have been synthesized by treatment of adamantane, its homologs, and structurally related cage hydrocarbons with nitric acid in acetic acid and subsequent addition of urea and heating.
-
Alkylation of adamantane with alkyl halides catalyzed by ruthenium complexes作者:R. I. Khusnutdinov、N. A. Schchadneva、A. I. Malikov、U. M. DzhemilevDOI:10.1134/s0965544106030030日期:2006.5The feasibility of catalytic alkylation of adamantane and 1-bromoadamantane with alkyl halides in the presence of ruthenium-containing catalysts was revealed. The optimum molar ratios between the catalyst components and the reactants, as well as the reaction conditions for the selective synthesis of mono- and dialkylsubstituted adamantane derivatives with a 70-98% yield, were determined.
-
Stepanov,F.N. et al., Journal of Organic Chemistry USSR (English Translation), 1969, vol. 5, p. 2123 - 2125作者:Stepanov,F.N. et al.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
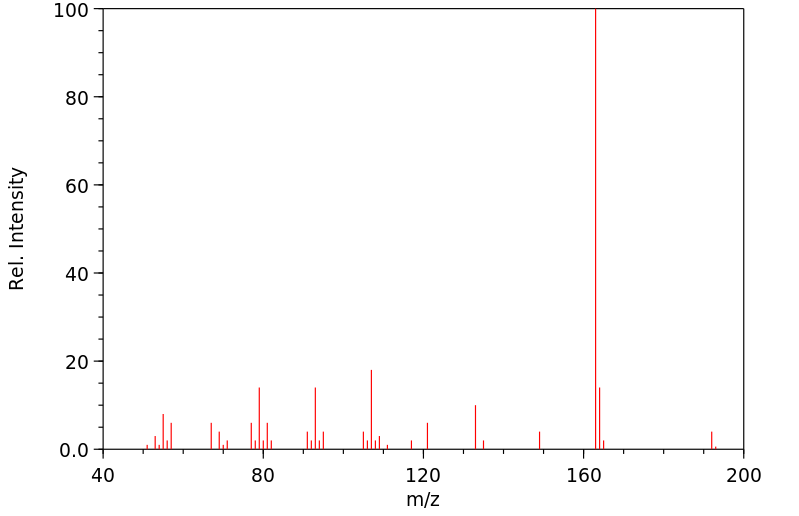
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
降冰片烯
金刚烷-D16
金刚烷
螺戊烷
螺二環己烷
螺[5.6]十二烷
螺[5.5]十一碳-4-烯
螺[5.2]辛-2-烯
螺[4.5]癸烷
螺[4.4]壬-8-烯
螺[3.4]辛烷
螺[3.4]辛-7-烯
螺[3.3]庚-2,5-二烯
螺[2.5]辛烷
螺[2.5]辛-7-烯
螺[2.5]辛-5,7-二烯
螺[2.4]庚-4,6-二烯
螺[2.4]庚-1-烯
螺[2.3]己-1-烯
螺[2.2]戊-1-烯
螺<二环<2.2.2>辛-5-烯-2,1'-环丙烷>
螺<4.4>壬-1,3,7-三烯
螺<4.4>壬-1,3,6,8-四烯
螺(4.4)壬烷
螺(4.4)壬-1,3-二烯
螺(3.4)辛-5,7-二烯
trans-perhydroazulene
萘烷
萘,1,2,3,4,4a,8a-六氢-,顺-
美罗培南中间体F9
篮烷
立方烷
氨基甲硫酸,二甲基-,O,O-(3,3-二甲基1,1-联苯基-2,2-二基)酯
棱晶烷
杜瓦苯
新戊基-1金刚烷
抗氧化剂TH-CPL
庚搭烯
四螺[2.0.2:0.2:0.2:0]十二烷
四螺[2.0.0.0.2.1.1.1]十一烷,顺-
四环己基铅
四环[5.3.0.0<2,6>.0<3,10>]癸-4,8-二烯
四环[5.3.0.0(2,6).0(3,10)]癸烷
四环[4.4.0.02,10.03,7]癸-4,8-二烯
四环[4.2.2.26,5.01,6]十二烷
四环[4.2.0.02,5.03,8]辛烷
四环[3.3.0.02,4.03,6]辛-7-烯
四环<5.3.1.02,6.04,9>十一烷
四环(8.2.2.22,5.26,9)十八碳-1,5,9-三烯
四环(4.1.0.0(2,4).0(3,5))庚烷