2,4-二氯苯氧丁酸 | 94-82-6
中文名称
2,4-二氯苯氧丁酸
中文别名
2,4-滴丁酸;2,4-D丁酸;4-(2,4-二氯苯氧基)丁酸;4-(2,4-二氯苯氧基)丁酸;2,4-DB
英文名称
4-(2,4-dichlorophenoxy)butyric acid
英文别名
2,4-DB;4-(2,4-dichlorophenoxy)butanoic acid;2,4-dichlorophenoxybutyric acid;2-(2,4-dichlorophenoxy)butyric acid;Butyrac;γ-(2,4-dichlorophenoxy)-butyric acid
CAS
94-82-6
化学式
C10H10Cl2O3
mdl
MFCD00002819
分子量
249.094
InChiKey
YIVXMZJTEQBPQO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:118-120 °C(lit.)
-
沸点:324.35 °C
-
密度:1.3342 (estimate)
-
物理描述:2,4-db appears as colorless crystals. Slightly corrosive to iron. A chlorinated phenoxy herbicide. Soluble in organic solvents.
-
颜色/状态:White crystals
-
气味:Slightly phenolic
-
溶解度:Water solubility: 46 ppm at 25 °C.
-
蒸汽压力:3.5X10-6 mm Hg at 25 deg (est)
-
分解:When heated to decomposition it emits toxic fumes of /hydrogen chloride/.
-
腐蚀性:The acid is slightly corrosive to iron.
-
解离常数:pKa= 4.95 at 25 °C
-
保留指数:1869;1826.1
-
稳定性/保质期:
避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):3.5
-
重原子数:15
-
可旋转键数:5
-
环数:1.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:46.5
-
氢给体数:1
-
氢受体数:3
ADMET
代谢
在动物和易感植物中通过β-氧化转化为2,4-D。
Converted by beta-oxidation to 2,4-D in animals and susceptible plants.
来源:Hazardous Substances Data Bank (HSDB)
代谢
大豆(Glycine max (L.) Merr. var. Lee)和苍耳(Xanthium sp.)含有beta-氧化酶酶,能够将2,4-DB降解为2,4-D。确定了一个中间代谢物为4-(2,4-二氯苯氧基)巴豆酸。另一代谢途径表明合成了10-(2,4-二氯苯氧基)癸酸。将2,4-DB(14)C应用于卷叶酸模(Rumex crispus L.)和细叶车前(Plantago lanceolata L.)。主要代谢途径是beta-氧化,2,4-D被确认为主要代谢物。其他未识别的代谢物似乎与极性脂质结合。将2,4-DB甲基酯混入大豆油中给予豚鼠。收集尿液,GLC-MS分析表明在用重氮甲烷处理提取物后存在2,4-D的甲基酯。大豆疫霉(Phytophthora megasprma var. sojae)和三叶草(Trifolium repens)的细胞悬浮液不能代谢2,4-D,但通过不包括beta-氧化的途径降解了2,4-DB。在21天的期间内,疫霉菌Phytophthora megasperma降解了约45%的2,4-DB。由于在营养培养基或真菌菌丝中未发现2,4-D,因此得出结论,降解不包括2,4-DB的beta-氧化。该真菌确实降解了2,4-D。在萨斯喀彻温省的土壤中,n-丁基2,4-DB酯的水解迅速,但未确定产物。
Soybean (Glycine max (L.) Merr. var. Lee) and cocklebur (Xanthium sp.) contained beta-oxidase enzymes that were capable of degrading 2,4-DB to 2,4-D. An intermediate metabolite was identified as 4-(2,4-dichlorophenoxy) crotonic acid. Another metabolite pathway was indicated by synthesis of 10-(2,4-dichlorophenoxy)decanoic acid. 2,4-DB(14)C was applied to curly dock (Rumex crispus L.) and buckhorn plantain (Plantago lanceolata L.). The main pathway of metabolism was beta-oxidation and 2,4-D was identified as the major metabolite. Other unidentified metabolites appeared bound to polar lipids. 2,4-DB methyl ester was administered in soybean oil to a guinea pig. Urine was collected and GLC-MS analysis indicated the presence of the methyl ester of 2,4-D after treatment of the extract with diazomethane. Phytophthora megasprma var. sojae and cell suspensions of white clover (Trifolium repens) were not able to metabolize 2,4-D but did degrade 2,4-DB by a path not including beta- oxidation. Over a 21-day period, the fungus Phytophthora megasperma degraded about 45% of the 2,4-DB present. Since no 2,4-D was found in the nutrient medium, or fungus myceluim, it was concluded that degradation did not include beta-oxidation of 2,4-DB. The fungus did degrade 2,4-D. In Saskatchewan soils, hydrolysis of n-butyl 2,4-DB ester was rapid but products were not identified.
来源:Hazardous Substances Data Bank (HSDB)
代谢
A typical beta-oxidation product (2,4-D) is observed via phenoxybutenoic acid in 2,4-DB metabolism by plants. The decanoic acid derivative of 2,4-dichlorophenol is an interesting metabolite.
来源:Hazardous Substances Data Bank (HSDB)
代谢
CDDs通过口服、吸入和皮肤暴露途径被吸收。CDDs通过血清脂质和脂蛋白在血浆中携带,主要分布到肝脏和脂肪组织。CDDs通过微粒体单加氧酶系统非常缓慢地代谢为极性代谢物,这些代谢物可以与葡萄糖醛酸和谷胱甘肽结合。它们可能通过诱导CDDs来增加自己的代谢速率,CDDs既诱导一期酶也诱导二期酶。CDDs的主要排泄途径是胆汁和粪便,尽管也有少量通过尿液和哺乳排出。
CDDs are absorbed through oral, inhalation, and dermal routes of exposure. CDDs are carried in the plasma by serum lipids and lipoproteins, distributing mainly to the liver and adipose tissue. CDDs are very slowly metabolized by the microsomal monooxygenase system to polar metabolites that can undergo conjugation with glucuronic acid and glutathione. They may increase the rate of their own metabolism by inducing CDDs induce both phase I and phase II enzymes. The major routes of excretion of CDDs are the bile and the feces, though smaller amounts are excreted in the urine and via lactation. (L177)
来源:Toxin and Toxin Target Database (T3DB)
毒理性
2,4-DB的一些内分泌效应可能是由2,4-D介导的性激素从性激素结合球蛋白上的位移,或者是2,4-D介导的阻断所需的OAT6运输蛋白,这些蛋白对于功能性有机离子和二羧酸盐(包括雌酮硫酸盐)的运输是必需的。
Some of the endocrine effects of 2,4-DB may be mediated by the 2,4-D mediated displacement of sex hormones from the sex hormone binding globulin or the 2,4-D mediated blocking or OAT6 transport proteins that are needed for the transport of functional organic ions and dicarboxylates (including estrone sulfate).
来源:Toxin and Toxin Target Database (T3DB)
毒理性
癌症分类:不太可能对人类致癌
Cancer Classification: Not Likely to be Carcinogenic to Humans
来源:Hazardous Substances Data Bank (HSDB)
毒理性
Classification of carcinogenicity: 1) evidence in humans: limited; Overall summary evaluation of carcinogenic risk to humans is Group 2B: The agent is possibly carcinogenic to humans. /Chlorophenoxy herbicides; From table/
来源:Hazardous Substances Data Bank (HSDB)
毒理性
2B,可能对人类有致癌性。
2B, possibly carcinogenic to humans. (L135)
来源:Toxin and Toxin Target Database (T3DB)
毒理性
所有形式的2,4-DB在通过皮肤吸收或吸入时被认为毒性较低。喂食中等剂量75毫克/千克的2,4-DB的雌性大鼠,经历了包括卵巢重量减轻、出生后代数量减少和整体体重下降等一系列慢性影响。此外,许多后代(幼崽)在哺乳期死亡。
All forms of 2,4-DB are considered low in toxicity when absorbed via skin or via inhalation. Female rats fed moderate doses of 75 mg/kg of 2,4-DB, experienced a number of chronic effects including lower ovarian weights, fewer offspring born and lower overall body weight. In addition, numerous offspring (pups) died during lactation.
来源:Toxin and Toxin Target Database (T3DB)
吸收、分配和排泄
酯类通常比其母酸具有更强的除草活性,这是因为它们能被目标植物更好地吸收。/苯氧基链烷酸/
ESTERS NORMALLY EXHIBIT GREATER HERBICIDAL ACTIVITY THAN PARENT ACIDS, BECAUSE OF IMPROVED ABSORPTION BY TARGET PLANTS. /PHENOXYALKANOIC ACIDS/
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
The chlorophenoxy compounds are absorbed across the gut wall, lung, and skin. They are not significantly fat storable. Excretion occurs almost entirely by way of the urine. /Chlorophenoxy herbicides/
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
危险等级:6.1(b)
-
危险品标志:Xn,N
-
安全说明:S25,S29,S46,S61
-
危险类别码:R22,R51/53
-
WGK Germany:2
-
海关编码:2918990021
-
危险品运输编号:UN 3077 9/PG 3
-
RTECS号:ES9100000
-
包装等级:III
-
危险类别:6.1(b)
-
储存条件:储存于阴凉、通风的库房,远离火种、热源,防止阳光直射。包装需密封,并与氧化剂分开存放,切忌混储。配备相应的消防器材。仓储区应准备合适的材料以处理泄漏物。
SDS
| 第一部分:化学品名称 |
| 化学品中文名称: | 2,4-滴丁酸;4-(2,4-二氯苯氧基)丁酸 |
| 化学品英文名称: | 2,4-DB;4-(2,4-Dichlorophenoxy)butanoic acid |
| 中文俗名或商品名: | |
| Synonyms: | |
| CAS No.: | 94-82-6 |
| 分子式: | C 10 H 10 Cl 2 O 9 |
| 分子量: | 249.10 |
| 第二部分:成分/组成信息 |
| 纯化学品 混合物 | ||||||
| 化学品名称:2,4-滴丁酸;4-(2,4-二氯苯氧基)丁酸 | ||||||
|
| 第三部分:危险性概述 |
| 危险性类别: | 第6.1类 毒害品 |
| 侵入途径: | 吸入 食入 |
| 健康危害: | 本品为中等毒除草剂。吸入、摄入或经皮肤吸收后对身体有害。对眼睛、皮肤和粘膜有刺激作用。 |
| 环境危害: | 对环境有危害,对水体可造成污染。 |
| 燃爆危险: | 本品可燃,具刺激性。 |
| 第四部分:急救措施 |
| 皮肤接触: | 用肥皂水及清水彻底冲洗。就医。 |
| 眼睛接触: | 提起眼睑,用流动清水或生理盐水冲洗。就医。 |
| 吸入: | 迅速脱离现场至空气新鲜处。保持呼吸道通畅。如呼吸困难,给输氧。如呼吸停止,立即进行人工呼吸。就医。 |
| 食入: | 误服者,饮适量温水,催吐。洗胃。就医。 |
| 第五部分:消防措施 |
| 危险特性: | 遇明火、高热可燃。其粉体与空气可形成爆炸性混合物, 当达到一定浓度时, 遇火星会发生爆炸。受高热分解放出有毒的气体。 |
| 有害燃烧产物: | 一氧化碳、二氧化碳、氯化氢。 |
| 灭火方法及灭火剂: | 雾状水、泡沫、干粉、二氧化碳、砂土。 |
| 消防员的个体防护: | 消防人员须佩戴防毒面具、穿全身消防服,在上风向灭火。 |
| 禁止使用的灭火剂: | |
| 闪点(℃): | |
| 自燃温度(℃): | |
| 爆炸下限[%(V/V)]: | |
| 爆炸上限[%(V/V)]: | |
| 最小点火能(mJ): | |
| 爆燃点: | |
| 爆速: | |
| 最大燃爆压力(MPa): | |
| 建规火险分级: |
| 第六部分:泄漏应急处理 |
| 应急处理: | 隔离泄漏污染区,周围设警告标志,建议应急处理人员戴好防毒面具,穿化学防护服。不要直接接触泄漏物,用砂土吸收,铲入提桶,倒至空旷地方深埋。也可以用大量水冲洗,经稀释的洗水放入废水系统。如大量泄漏,收集回收或无害处理后废弃。 |
| 第七部分:操作处置与储存 |
| 操作注意事项: | 密闭操作,局部排风。防止粉尘释放到车间空气中。操作人员必须经过专门培训,严格遵守操作规程。建议操作人员佩戴自吸过滤式防尘口罩,戴化学安全防护眼镜,穿防毒物渗透工作服,戴橡胶手套。远离火种、热源,工作场所严禁吸烟。使用防爆型的通风系统和设备。避免产生粉尘。避免与氧化剂接触。配备相应品种和数量的消防器材及泄漏应急处理设备。倒空的容器可能残留有害物。 |
| 储存注意事项: | 储存于阴凉、通风的库房。远离火种、热源。防止阳光直射。包装密封。应与氧化剂分开存放,切忌混储。配备相应品种和数量的消防器材。储区应备有合适的材料收容泄漏物。 |
| 第八部分:接触控制/个体防护 |
| 最高容许浓度: | 中 国 MAC:未制订标准前苏联 MAC:未制订标准美国TLV—TWA:未制订标准 |
| 监测方法: | |
| 工程控制: | 密闭操作,局部排风。 |
| 呼吸系统防护: | 空气中粉尘浓度超标时,必须佩戴自吸过滤式防尘口罩。紧急事态抢救或撤离时,应该佩戴空气呼吸器。 |
| 眼睛防护: | 戴化学安全防护眼镜。 |
| 身体防护: | 穿防毒物渗透工作服。 |
| 手防护: | 戴橡胶手套。 |
| 其他防护: | 工作现场禁止吸烟、进食和饮水。工作后,淋浴更衣。单独存放被毒物污染的衣服,洗后再用。注意个人清洁卫生 |
| 第九部分:理化特性 |
| 外观与性状: | 白色结晶。 |
| pH: | |
| 熔点(℃): | 117~119 |
| 沸点(℃): | |
| 相对密度(水=1): | |
| 相对蒸气密度(空气=1): | |
| 饱和蒸气压(kPa): | |
| 燃烧热(kJ/mol): | |
| 临界温度(℃): | |
| 临界压力(MPa): | |
| 辛醇/水分配系数的对数值: | |
| 闪点(℃): | |
| 引燃温度(℃): | |
| 爆炸上限%(V/V): | |
| 爆炸下限%(V/V): | |
| 分子式: | C 10 H 10 Cl 2 O 9 |
| 分子量: | 249.10 |
| 蒸发速率: | |
| 粘性: | |
| 溶解性: | 难溶于水,易溶于多数有机溶剂。 |
| 主要用途: | 用作农用除草剂。 |
| 第十部分:稳定性和反应活性 |
| 稳定性: | 在常温常压下 稳定 |
| 禁配物: | 强氧化剂。 |
| 避免接触的条件: | |
| 聚合危害: | 不能出现 |
| 分解产物: | 一氧化碳、二氧化碳、氯化氢。 |
| 第十一部分:毒理学资料 |
| 急性毒性: | LD50:700mg/kg(大鼠经口);800mg/kg(大鼠经皮) LC50: |
| 急性中毒: | |
| 慢性中毒: | |
| 亚急性和慢性毒性: | |
| 刺激性: | |
| 致敏性: | |
| 致突变性: | |
| 致畸性: | |
| 致癌性: |
| 第十二部分:生态学资料 |
| 生态毒理毒性: | |
| 生物降解性: | |
| 非生物降解性: | |
| 生物富集或生物积累性: |
| 第十三部分:废弃处置 |
| 废弃物性质: | |
| 废弃处置方法: | |
| 废弃注意事项: |
| 第十四部分:运输信息 |
| |
| 危险货物编号: | 61890 |
| UN编号: | 2765 |
| 包装标志: | |
| 包装类别: | |
| 包装方法: | 塑料袋或二层牛皮纸袋外全开口或中开口钢桶;两层塑料袋或一层塑料袋外麻袋、塑料编织袋、乳胶布袋;塑料袋外复合塑料编织袋(聚丙烯三合一袋、聚乙烯三合一袋、聚丙烯二合一袋、聚乙烯二合一袋);塑料袋或二层牛皮纸袋外普通木箱;螺纹口玻璃瓶、塑料瓶、复合塑料瓶或铝瓶外普通木箱;塑料瓶、两层塑料袋或两层牛皮纸袋(内或外套以塑料袋)外瓦楞纸箱。 |
| 运输注意事项: | 铁路运输时包装所用的麻袋、塑料编织袋、复合塑料编织袋的强度应符合国家标准要求。运输前应先检查包装容器是否完整、密封,运输过程中要确保容器不泄漏、不倒塌、不坠落、不损坏。严禁与酸类、氧化剂、食品及食品添加剂混运。运输时运输车辆应配备相应品种和数量的消防器材及泄漏应急处理设备。运输途中应防曝晒、雨淋,防高温。公路运输时要按规定路线行驶,勿在居民区和人口稠密区停留。 |
| RETCS号: | |
| IMDG规则页码: |
| 第十五部分:法规信息 |
| 国内化学品安全管理法规: | 化学危险物品安全管理条例 (1987年2月17日国务院发布),化学危险物品安全管理条例实施细则 (化劳发[1992] 677号),工作场所安全使用化学品规定 ([1996]劳部发423号)等法规,针对化学危险品的安全使用、生产、储存、运输、装卸等方面均作了相应规定;常用危险化学品的分类及标志 (GB 13690-92)将该物质划为第6.1 类毒害品。 |
| 国际化学品安全管理法规: |
| 第十六部分:其他信息 |
| 参考文献: | 1.周国泰,化学危险品安全技术全书,化学工业出版社,1997 2.国家环保局有毒化学品管理办公室、北京化工研究院合编,化学品毒性法规环境数据手册,中国环境科学出版社.1992 3.Canadian Centre for Occupational Health and Safety,CHEMINFO Database.1998 4.Canadian Centre for Occupational Health and Safety, RTECS Database, 1989 |
| 填表时间: | 年月日 |
| 填表部门: | |
| 数据审核单位: | |
| 修改说明: | |
| 其他信息: | 5 |
| MSDS修改日期: | 年月日 |
制备方法与用途
化学性质
纯品为无色油状液体。沸点169℃/266Pa,熔点9℃,闪点48℃以上,相对密度1.2428。易溶于多种有机溶剂,难溶于水,挥发性强。对酸、热稳定,遇碱分解为2,4-滴钠盐及丁醇。工业品呈棕色,有酚臭味。
用途
广谱性、激素型除草剂,具有良好的展着性和内吸性。主要用于水田和麦田等,主要防除禾本科作物田中的双子叶杂草、异形莎科及某些恶性杂草,如鸭舌草、眼子菜、小三棱草、蓼、看麦娘、豚草、野苋、藜等。
生产方法
2,4-滴的合成:首先合成苯酚钠与氯乙酸钠,然后两者缩合。反应在110~120℃下进行,并维持溶液微碱性。一氯醋酸与苯酚投料比为1:1.16。之后进行氯化反应,以氯气作为氯化剂,在92~98℃下反应。另一种操作方法是:将工业用苯酚31.6g(0.332mol)和适量甲苯混合后升温至接近回流点,同时滴加氢氧化钠和氯乙酸水溶液,滴加时间约1小时,在回流温度反应1小时。反应完毕加入230mL自来水分出有机相溶剂循环使用,得苯氧基乙酸,无需蒸馏操作,直接进行氯化反应。以氯气为氯化剂,并添加微量催化剂(如碘粉),氯化时间1~3.5小时,在65~90℃下进行。氯化结束在室温下过滤洗涤干燥,最终得到2,4-D 57.0g,两步总收率≥76%,产品质量明显提高。
2,4-滴丁酯的合成:将湿2,4-滴在搅拌下加入定量丁醇中,升温至120~140℃,保持4小时充分脱水。待分离器水面不再上升时停止回流,继续加热至160~170℃,减压蒸出丁醇。
类别
农药
毒性分级
中毒
急性毒性
口服-大鼠 LD50: 700 毫克/公斤
可燃性危险特性
燃烧产生有毒氯化物气体
储运特性
库房通风低温干燥;与食品原料分开储存运输
灭火剂
干粉、泡沫、砂土
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 2,4-dichlorophenoxybutanol 60222-61-9 C10H12Cl2O2 235.11 —— ethyl 2,4-dichlorophenoxybutanoate 42609-41-6 C12H14Cl2O3 277.147 4-(2,4-二氯苯氧基)丁酸丁酯 2,4-DB butyl ester 6753-24-8 C14H18Cl2O3 305.2 4-(2,4-二氯苯氧基)丁腈 4-(2,4-dichlorophenoxy)butanenitrile 63867-25-4 C10H9Cl2NO 230.094 (3-氯-丙基)-(2,4-二氯-苯基)-醚 (3-chloro-propyl)-(2,4-dichloro-phenyl)-ether 78483-28-0 C9H9Cl3O 239.529 1-(3-溴丙氧基)-2,4-二氯苯 1-(3-bromopropoxy)-2,4-dichlorobenzene 6954-78-5 C9H9BrCl2O 283.98 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— ethyl 2,4-dichlorophenoxybutanoate 42609-41-6 C12H14Cl2O3 277.147 —— 4-(2,4-dichloro-phenoxy)-butyryl chloride 31770-28-2 C10H9Cl3O2 267.539 —— 4-(2,4-dichloro-phenoxy)-butyric acid amide 51992-35-9 C10H11Cl2NO2 248.109 —— 4-(2,4-dichlorophenoxy)-N-phenylbutanamide —— C16H15Cl2NO2 324.2 2甲4氯丁酸 MCPB 94-81-5 C11H13ClO3 228.675
反应信息
-
作为反应物:描述:2,4-二氯苯氧丁酸 以56%的产率得到参考文献:名称:ZEIGER G.; HEILMANN D .; HENNING D.; KEMPTER G., WISS. Z. PAED. HOCH. K. LIEBKNECHT POTSDAM (TEIL 1), 1977, 21, NO 1, 29-4+摘要:DOI:
-
作为产物:描述:4-phenoxybutan-1-ol 在 3,4-二氯噻吩 、 platinum on activated charcoal 、 氯 、 zinc(II) chloride 作用下, 以 水 为溶剂, 85.0 ℃ 、1.1 MPa 条件下, 反应 0.5h, 生成 2,4-二氯苯氧丁酸参考文献:名称:一种氯代苯氧羧酸的制备方法摘要:本发明提供了一种氯代苯氧羧酸的制备方法,包括以下步骤:S1)苯氧脂肪醇在催化剂A和催化剂B的作用下,和氯化剂进行2位和/或4位的选择性氯化反应,得到氯代苯氧脂肪醇;所述催化剂A为路易斯酸;所述催化剂B为C5~22的硫醚、噻唑、异噻唑、噻吩或它们的卤代衍生物;S2)氯代苯氧脂肪醇和水,在催化剂的作用下,和氧化剂进行催化氧化反应,得到氯代苯氧羧酸。本发明通过对工艺路线的重新设计,对催化剂和氯化剂的精细筛选,有效降低了能耗,提高了氯化选择性同时避免了有效成分的损失,所得氯代苯氧羧酸的含量可达98.5%以上,总收率可达99%以上。公开号:CN108947799A
文献信息
-
Piperazine and homopiperazine compounds
-
Novel camptothecin derivatives. part 1: oxyalkanoic acid esters of camptothecin and their in vitro and in vivo antitumor activity作者:Li-Xi Yang、Xiandao Pan、Hui-Juan WangDOI:10.1016/s0960-894x(02)00153-1日期:2002.5cells in vitro by the colony formation assay and in vivo. These newly synthesized derivatives show a dramatically higher chemotherapeutic activity in killing human cancer cells than the parent drug, camptothecin, and clinically available drugs, irinotecan and taxol.
-
Automated Synthesis and Purification of Amides: Exploitation of Automated Solid Phase Extraction in Organic Synthesis作者:R. Michael Lawrence、Scott A. Biller、Olga M. Fryszman、Michael A. PossDOI:10.1055/s-1997-1232日期:1997.5Automated parallel synthesis of small organic molecules, either as single entities or as mixtures, offers the potential for the rapid optimization of physical and biological properties of a molecule. Currently, emphasis has been placed on solid phase synthesis technology to accomplish the rapid preparation of large numbers of molecules. Automated solution phase synthesis is an alternative approach which has the advantages of having shorter development times and being more amenable to scaleup. Utilizing commercially available liquid handlers for reaction setup and exploiting automated solid phase extraction for product purification, a procedure has been developed to prepare and purify up to 100 amide analogs, simultaneously. Both carbodiimide mediated couplings and p-nitrophenyl ester displacements have been carried out using this procedure. Product amides having overall neutral or basic character have been prepared in good yield and with good to excellent purities.
-
HERBICIDALLY ACTIVE 3-PHENYLISOXAZOLINE-5-CARBOXAMIDES OF TETRAHYDRO- AND DIHYDROFURANCARBOXAMIDES申请人:BAYER AKTIENGESELLSCHAFT公开号:US20200216403A1公开(公告)日:2020-07-09The invention relates to 3-phenylisoxazoline-5-carboxamides of tetrahydro and dihydrofuran carboxamides of general formula (I) and to their agrochemically compatible salts (I) as well as to the use thereof in the field of plant protection.这项发明涉及一般式(I)的3-苯基异噁唑啉-5-羧酰胺以及其农药兼容盐(I),以及它们在植物保护领域中的应用。
-
SUBSTITUTED 3-HETEROARYLOXY-1H-PYRAZOLES AND SALTS THEREOF AND THEIR USE AS HERBICIDAL ACTIVE SUBSTANCES申请人:BAYER CROPSCIENCE AKTIENGESELLSCHAFT公开号:US20200181117A1公开(公告)日:2020-06-11A substituted 3-heteroaryloxy-1H-pyrazole of the general formula (I) or salt thereof Substituted 3-heteroaryloxy-1H-pyrazoles of the general formula (I) are described, as is their use as herbicides, in particular for controlling broad-leaved weeds and/or weed grasses in crops of useful plants and/or as plant growth regulators for influencing the growth of crops of useful plants described. The present invention also relates to herbicidal and/or plant growth-regulating compositions comprising one or more compounds of the general formula (I).
表征谱图
-
氢谱1HNMR
-
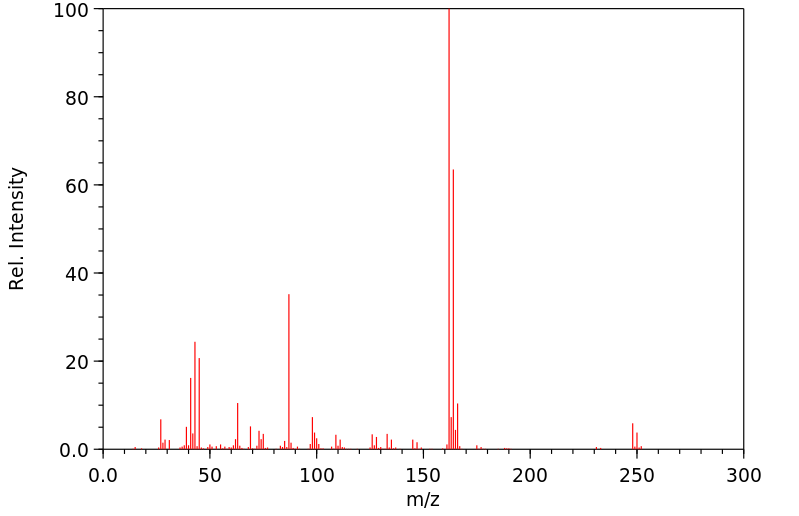
质谱MS
-
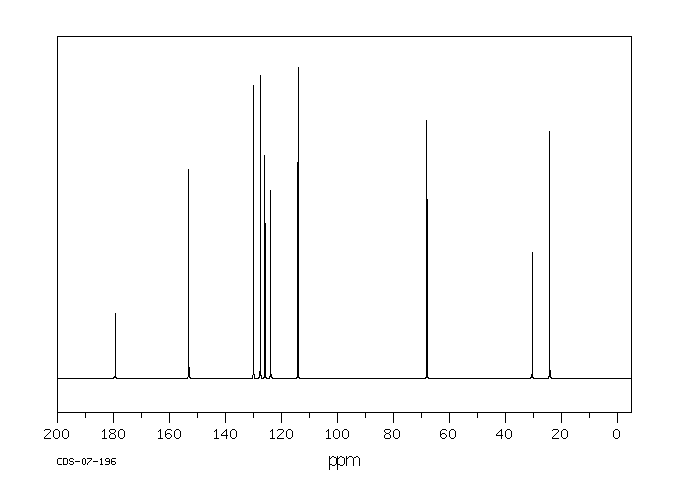
碳谱13CNMR
-
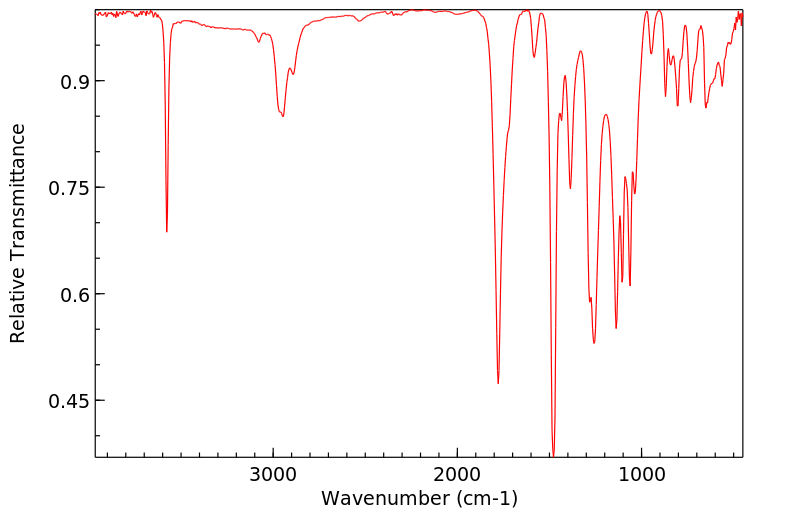
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫