3-氨基-N-乙基哌啶 | 6789-94-2
中文名称
3-氨基-N-乙基哌啶
中文别名
1-乙基哌啶-3-胺;1-乙基-3-哌啶氨;N-乙基-3-氨基哌啶;3-氨基-N-乙基六氢吡啶
英文名称
1-ethylpiperidin-3-amine
英文别名
1-ethyl-3-piperidinamine;(rac)-(1-ethyl-3-piperidyl)amine;1-ethyl-3-amino-piperidine;3-amino-N-ethylpiperidine;1-ethyl-piperidin-3-ylamine;3-amino-1-ethyl piperidine;1-Aethyl-[3]piperidylamin;1-ethylpiperidine-3-amine
CAS
6789-94-2
化学式
C7H16N2
mdl
MFCD00044587
分子量
128.217
InChiKey
WAKUKXKZEXFXJP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:155℃
-
密度:0.898
-
闪点:61℃
-
溶解度:甲醇
计算性质
-
辛醇/水分配系数(LogP):0.2
-
重原子数:9
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:29.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
海关编码:2933399090
-
储存条件:20°C
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— N-(1-ethyl-[3]piperidyl)-acetamide 24059-81-2 C9H18N2O 170.255
反应信息
-
作为反应物:描述:3-氨基-N-乙基哌啶 在 盐酸 作用下, 以 乙醇 为溶剂, 反应 20.25h, 生成 N2-(3,4-dichlorophenyl)-N4-(1-ethyl-3-piperidinyl)-2,4-quinazolinediamine dihydrochloride参考文献:名称:N 2-芳基-N 4-[(二烷基氨基)烷基]-和N 4-芳基-N 2-[(二烷基氨基)烷基] -2,4-喹唑啉二胺的合成和抗疟作用。摘要:合成了一系列的N2(和N4)-芳基-N4(和N2)-[((二烷基氨基)烷基] -2,4-喹唑啉二胺类,用于抗疟疾评估。适当的2,4-二氯喹唑啉(IV)与必要的N,N-二烷基亚烷基二胺缩合得到一系列的2-氯-N-[((二烷基氨基)烷基] -4-喹唑啉胺(V),其与适当的芳基胺缩合。提供相应的N 2-芳基-N 4-[((二烷基氨基)烷基] -2,4-喹唑啉二胺(VI)。将2,4-二氯喹唑啉水解为2-氯-4-喹唑啉,然后与适当的N,N-二烷基亚烷基二胺缩合,得到2-[[[(二烷基氨基)烷基]氨基] -4-喹唑啉(IXa)阵列。用三氯氧化磷氯化并与必要的芳基胺缩合得到N2-[((二烷基氨基)烷基] -N4-苯基-2,4-喹唑啉二胺(X)。N2-芳基-N4-[((二烷基氨基)烷基] -2,4-喹唑啉二胺(VI)中普遍具有抗疟活性,而反向异构体的活性较低。光毒性责任排除了对该系列成员的临床评估。DOI:10.1021/jm00134a002
-
作为产物:描述:1-Ethyl-piperidin-3-one oxime 在 红铝 作用下, 生成 3-氨基-N-乙基哌啶参考文献:名称:A novel series of 6-methoxy-1H-benzotriazole-5-carboxamide derivatives with dual antiemetic and gastroprokinetic activities摘要:A novel series of 6-methoxy-1H-benzotriazole-5-carboxamide derivatives with a medium perhydroazacycle ring in the amine moiety were prepared, and their antiemetic and gastroprokinetic activities were evaluated. Among them, N-(1-ethylhexahydroazepin-3-yl)-, N-(1-ethyloctahydroazocin-3-yl)- and N-(1-ethyloctahydroazonin-3-yl)-6-methoxy-1H-benzotriazole-5-carboxamides (24, 36, 37) showed a potent antiemetic activity (inhibition of apomorphine-induced emesis in dogs) along with gastroprokinetic activity (gastric emptying in rats). (C) 1998 Elsevier Science Ltd. All rights reserved.DOI:10.1016/s0960-894x(98)00341-2
文献信息
-
Dual Pharmacophores - PDE4-Muscarinic Antagonistics申请人:Callahan James Francis公开号:US20090197871A1公开(公告)日:2009-08-06The present invention is directed to novel compounds of Formula (I), pharmaceutical compositions and their use in therapy, for example as inhibitors of phosphodiesterase type IV (PDE4) and as antagonists of muscarinic acetylcholine receptors (mAChRs), in the treatment of/and or prophylaxis of respiratory diseases, including antiinflammatory and/or allergic diseases such as chronic obstructive pulmonary disease (COPD), asthma, rhinitis (e.g. allergic rhinitis), atopic dermatitis or psoriasis.
-
Pyrrolidine derivatives申请人:——公开号:US20020049243A1公开(公告)日:2002-04-25The present invention relates to pyrrolidine derivatives and dimeric forms and/or pharmaceutically acceptable esters, and/or salts thereof. The compounds are useful as inhibitors of metalloproteases, e.g. zinc proteases, particularly zinc hydrolases, and which are effective in treating disease states are associated with vasoconstriction of increasing occurrences.
-
[EN] BICYCLIC INHIBITORS OF PAD4<br/>[FR] INHIBITEURS BICYCLIQUES DE PAD4
-
[EN] USE OF AND SOME NOVEL IMIDAZOPYRIDINES<br/>[FR] NOUVELLES IMIDAZOPYRIDINES ET LEUR UTILISATION申请人:ASTRAZENECA AB公开号:WO2004016611A1公开(公告)日:2004-02-26The use of compounds of formula (I) wherein R1, R3, R10, m and Ar are as defined in the Specification and pharmaceutically acceptable salts thereof in the manufacture of a medicament for the treatment or prophylaxis of diseases or conditions in which inhibition of kinase Itk activity is beneficial is disclosed. Certain novel compounds of formula (I), together with processes for their preparation, compositions containing them and their use in therapy are also disclosed.公开了在制备药物用于治疗或预防抑制激酶Itk活性有益的疾病或症状中,使用式(I)中R1、R3、R10、m和Ar所定义的化合物及其药学上可接受的盐。还公开了式(I)的某些新化合物,以及它们的制备方法、含有它们的组合物和它们在治疗中的用途。
-
Fused imidazoheterocyclic compounds and pharmaceutical compositions申请人:A. H. Robins Company, Inc.公开号:US04772600A1公开(公告)日:1988-09-20Imidazoheterocyclic compounds having the formula: ##STR1## wherein the A ring is pyridine in any of its four positions; B is carbonyl, thioxomethyl or hydroxymethylene; Z is hydrogen, halogen, loweralkyl, hydroxy, loweralkoxy, diloweralkylamino or nitro; Ar is phenyl, pyrido, thienyl or furanyl; and W forms a wide combination of groups with B, including acids, esters, alcohols, amides and ketones. The compounds have CNS activity in a method for treating a living animal body as muscle relaxants, anticonvulsants and antianxiety agents.
表征谱图
-
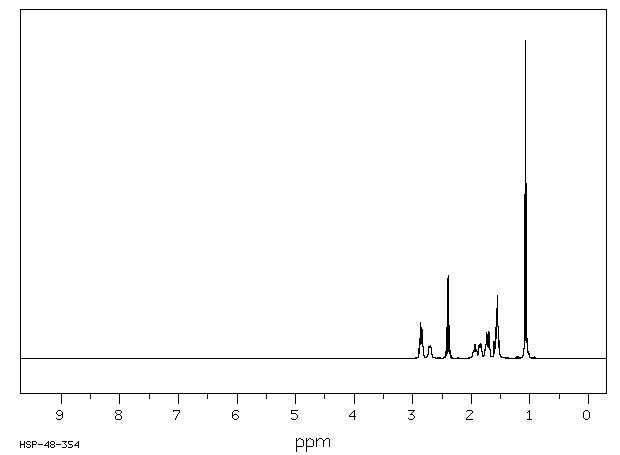
氢谱1HNMR
-
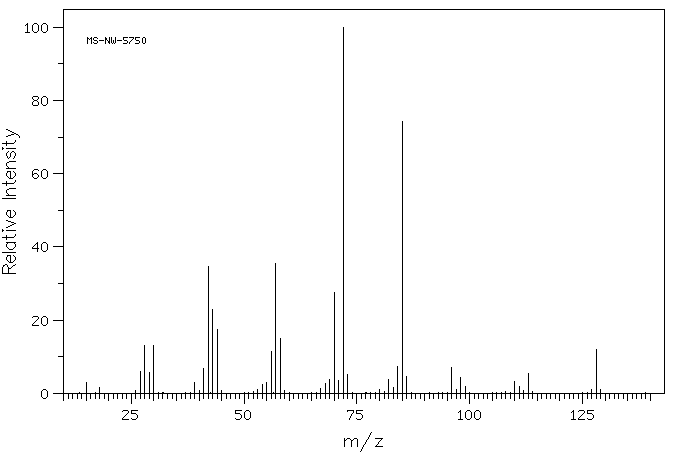
质谱MS
-
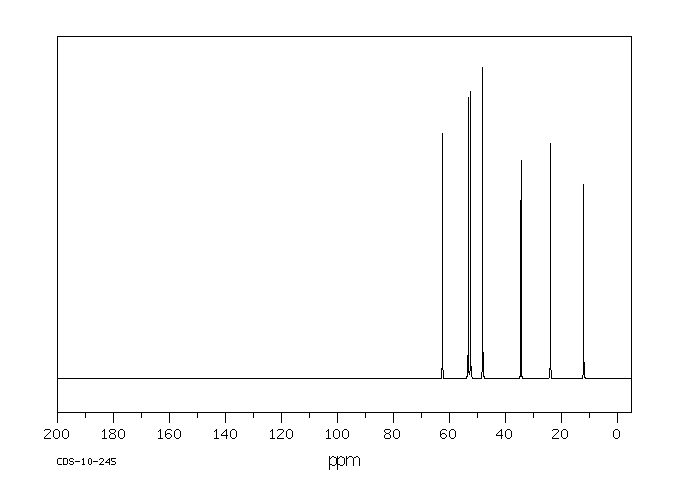
碳谱13CNMR
-
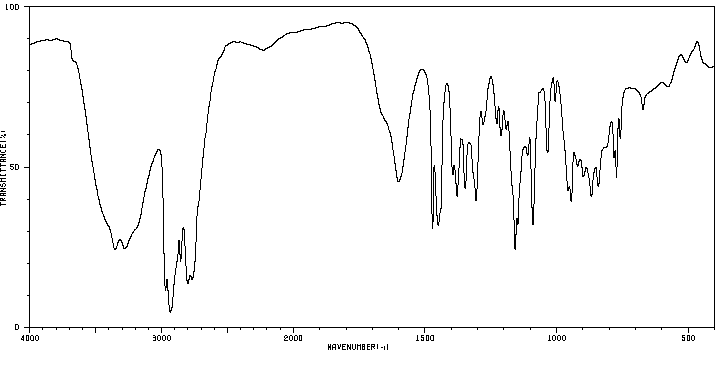
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-甲基哌啶盐酸盐;
(R)-2-苄基哌啶-1-羧酸叔丁酯
((3S,4R)-3-氨基-4-羟基哌啶-1-基)(2-(1-(环丙基甲基)-1H-吲哚-2-基)-7-甲氧基-1-甲基-1H-苯并[d]咪唑-5-基)甲酮盐酸盐
高氯酸哌啶
高托品酮肟
马来酸帕罗西汀
颜料红48:4
顺式3-氟哌啶-4-醇盐酸盐
顺式2,6-二甲基哌啶-4-酮
顺式1-苄基-4-甲基-3-甲氨基-哌啶
顺式-叔丁基4-羟基-3-甲基哌啶-1-羧酸酯
顺式-6-甲基-哌啶-1,3-二甲酸1-叔丁酯
顺式-5-(三氟甲基)哌啶-3-羧酸甲酯盐酸盐
顺式-4-叔丁基-2-甲基哌啶
顺式-4-Boc-氨基哌啶-3-甲酸甲酯
顺式-4-(氮杂环丁烷-1-基)-3-氟哌
顺式-3-顺式-4-氨基哌啶
顺式-3-甲氧基-4-氨基哌啶
顺式-3-BOC-3,7-二氮杂双环[4.2.0]辛烷
顺式-3-(1-吡咯烷基)环丁腈
顺式-3,5-哌啶二羧酸
顺式-3,4-二溴-3-甲基吡咯烷盐酸盐
顺式-2,6-二甲基-4-氧代哌啶-1-羧酸叔丁基酯
顺式-1-叔丁氧羰基-4-甲基氨基-3-羟基哌啶
顺式-1-boc-3,4-二氨基哌啶
顺式-1-(4-叔丁基环己基)-4-苯基-4-哌啶腈
顺式-1,3-二甲基-4-乙炔基-6-苯基-3,4-哌啶二醇
顺-4-(4-氟苯基)-1-(4-异丙基环己基)-4-哌啶羧酸
顺-4-(2-氟苯基)-1-(4-异丙基环己基)-4-哌啶羧酸
顺-3-氨基-4-氟哌啶-1-羧酸叔丁酯
顺-1-苄基-4-甲基哌啶-3-氨基酸甲酯盐酸盐
非莫西汀
雷芬那辛
雷拉地尔
阿维巴坦中间体4
阿格列汀杂质
阿尼利定盐酸盐 CII
阿尼利定
阿塔匹酮
阿哌沙班杂质BMS-591455
阿哌沙班杂质87
阿哌沙班杂质52
阿哌沙班杂质51
阿哌沙班杂质5
阿哌沙班杂质
阿哌沙班杂质
阿哌沙班-d3
阿哌沙班
阻聚剂701
间氨基谷氨酰胺