4-chloro-2-methylthiophenol | 17178-01-7
中文名称
——
中文别名
——
英文名称
4-chloro-2-methylthiophenol
英文别名
4-chloro-2-methylbenzenethiol;4-Chlor-2-methyl-thiophenol
CAS
17178-01-7
化学式
C7H7ClS
mdl
MFCD00041469
分子量
158.652
InChiKey
YIUPEFSJLWXHJR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:117 °C(Press: 21 Torr)
-
密度:1.217±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:9
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:1
-
氢给体数:1
-
氢受体数:1
安全信息
-
海关编码:2930909090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-[(4-氯-2-甲基苯基)硫代]-乙酸 (4-chloro-2-methyl-phenylsulfanyl)-acetic acid 94-76-8 C9H9ClO2S 216.688 3-氯甲苯 1-chloro-3-methylbenzene 108-41-8 C7H7Cl 126.586 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-[(4-氯-2-甲基苯基)硫代]-乙酸 (4-chloro-2-methyl-phenylsulfanyl)-acetic acid 94-76-8 C9H9ClO2S 216.688
反应信息
-
作为反应物:描述:4-chloro-2-methylthiophenol 在 磷酸酐 、 N-溴代丁二酰亚胺(NBS) 、 磷酸 、 sodium 、 potassium carbonate 、 过氧化苯甲酰 作用下, 以 四氯化碳 、 N,N-二甲基甲酰胺 为溶剂, 25.0~180.0 ℃ 、2.0 kPa 条件下, 反应 4.5h, 生成 (E)-N-[(5-chloro-1-benzothiophen-7-yl)methyl]-N,6,6-trimethylhept-2-en-4-yn-1-amine参考文献:名称:Synthesis and structure-activity relationships of benzo[b]thienylallylamine antimycotics摘要:Benzo[b]thiophene analouges of the allylamine antimycotic terbinafine (2) bearing the side chain at various positions and optionally substituted by halogen have been prepared and their antifungal activity studied. Derivatives bearing the side chain at positions 3, 4, or 7 are bioequivalents of 2. Compounds containing the allylamine side chain at position 7, with a further substituent at position 3, showed significantly enhanced activity against Candida albicans, an effect which appears to be specifically linked only to this particular substitution pattern. 3-Chloro-7-benzo[b]thienyl derivative 7m was found to be the most potent allylamine antimycotic identified so far. In general, substituted benzo[b]thiophenes can be used not only as potential equivalents of naphthalene in bioactive compounds but also as a tool to selectively modify biological activities.DOI:10.1021/jm00105a011
-
作为产物:描述:参考文献:名称:US1897516摘要:公开号:
文献信息
-
Process for phenol alkylthiolation and its application to the synthesis申请人:Societe Nationale Elf Aquitaine (Production)公开号:US05113019A1公开(公告)日:1992-05-12The invention relates to the preparation of alkylthiophenols by reaction of a dialkyl disulphide with a phenol. In the process according to the invention, the reaction is carried out in the presence of aluminum chloride or of ferric chloride in a solvent of the alkylbenzene type or, soley in the case of methylthiolation, in an excess of dimethyl disulphide. This process makes it possible, in particular to obtain, with a selectivity and in a yield which are excellent, 2-alkylthiophenols which may then be converted into 4-acyl-2-alkylthiophenols by means of a reaction at a temperature ranging from 40.degree. to 100.degree. C. with a complex BF.sub.3 :2RCOOH where R denotes an alkyl or propenyl radical, in a proportion of 10 to 15 moles of complex per mole of 2-alkylthiophenol.
-
NOVEL CYCLOPENTANE DERIVATIVES申请人:Banner David公开号:US20100317647A1公开(公告)日:2010-12-16The invention relates to a compound of formula (I) wherein A 1 and R 1 to R 5 are defined as in the description and in the claims. The compound of formula (I) can be used as a medicament.
-
[EN] SYNTHETIC PENTASACCHARIDES HAVING SHORT HALF-LIFE AND HIGH ACTIVITY<br/>[FR] PENTASACCHARIDES SYNTHÉTIQUES AYANT UNE DEMI-VIE COURTE ET UNE ACTIVITÉ ÉLEVÉE申请人:ENDOTIS PHARMA公开号:WO2012172104A1公开(公告)日:2012-12-20The invention concerns a pentasaccharide compound of formula (I) and the salts thereof. The invention also concerns a pharmaceutical composition comprising the synthetic pentasaccharide compound of formula (I) and its salts. The invention further concerns these compounds for use as a medicament, and in particular intended to treat blood clotting disorders, to prevent ischaemia reperfusion injury associated with solid organ transplantation, or to reduce the risk of blood clotting in an extracorporeal blood circuit during cardiac surgery, extracorporeal membrane oxygenation, or during circulatory assistance such as artificial heart.这项发明涉及一种化学式(I)及其盐的五糖化合物。该发明还涉及一种包括合成五糖化合物及其盐的药物组合物。此外,该发明还涉及这些化合物作为药物的用途,特别是用于治疗血液凝块疾病,预防固体器官移植相关的缺血再灌注损伤,或在心脏手术、体外膜肺氧合或人工心脏等循环辅助过程中减少体外循环血路中的血液凝块风险。
-
[EN] 3-(PHENYLSULFONYL)-[1,2,3]TRIAZOLO[1,5A]QUINAZOLIN-5(4H)-ONE DERIVATIVES<br/>[FR] DÉRIVÉS DE 3-(PHÉNYLSULFONYL)- [1,2,3]TRIAZOLO[1,5A]QUINAZOLIN-5(4H)-ONE申请人:BIOVERSYS AG公开号:WO2020109350A1公开(公告)日:2020-06-04The present invention relates to a compound according to formula (I), wherein R1 and R5 are independently selected from H, halogen, hydroxyl, NO2, CN, C1-C6-alkyl optionally substituted by one or more R11, C1-C6-alkoxy optionally substituted by one or more R11, C3-C6-cycloalkyl optionally substituted by one or more R11, -Cn-alkyl-N(R12)(R13) with n=0-3, -Cn-alkyl-C(O)N(R12)(R13) with n=0-3, -SO2-N(R14)-C(O)-R15; -Cn-alkyl-N(R14)-C(O)-R15 with n=0-3, -Cn-alkyl-C(O)-OR16 with n=0-3, -O(C1-C3-alkyl-O)m-C1-C3-alkyl-OR10 with m=0-3, -Cn-alkyl-OR16 with n=0-3, -NH-Cn-alkyl-R18 with n=0-3; -O-Cn-alkyl-R18 with n=0-3; -OPO(OR10)2, -PO(OR10)2, and a heterocycle optionally substituted by one or more R17; R3 is selected from halogen, hydroxyl, NO2, CN, C1-C6-alkyl optionally substituted by one or more R11, C1-C6-alkoxy optionally substituted by one or more R11, C3-C6-cycloalkyl optionally substituted by one or more R11, -Cn-alkyl-N(R12)(R13) with n=0-3, -Cn-alkyl-C(O)N(R12)(R13) with n=0-3, -SO2-N(R14)-C(O)-R15; -Cn-alkyl-N(R14)-C(O)-R15 with n=0-3, -Cn-alkyl-C(O)-OR16 with n=0-3, -O(C1-C3-alkyl-O)m-C1-C3-alkyl-OR10 with m=0-3, -Cn-alkyl-OR16 with n=0-3, -NH-Cn-alkyl-R18 with n=0-3; -O-Cn-alkyl-R18 with n=0-3; -OPO(OR10)2, -PO(OR10)2, and a heterocycle optionally substituted by one or more R17; R2 and R4 are independently selected from H, halogen, C1-C6-alkyl optionally substituted by one or more R11; R6, R7, R8 and R9 are independently selected from H, halogen, hydroxyl, NO2, CN, C1-C6-alkyl optionally substituted by one or more R11, C1-C6-alkoxy optionally substituted by one or more R11, C3-C6-cycloalkyl optionally substituted by one or more R11, -Cn-alkyl-N(R12)(R13) with n=0-3, -Cn-alkyl-C(O)N(R12)(R13) with n=0-3, -SO2-N(R12)(R13), -SO2-N(R14)-C(O)-R15; -Cn-alkyl-N(R14)-C(O)-R15 with n=0-3, -Cn-alkyl-C(O)-OR16 with n=0-3, -O(C1-C3-alkyl-O)m-C1-C3-alkyl-OR10 with m=0-3, -Cn-alkyl-OR16 with n=0-3, -NH-Cn-alkyl-R18 with n=0-3; -O-Cn-alkyl-R18 with n=0-3; -OPO(OR10)2, -PO(OR10)2, and a heterocycle optionally substituted by one or more R17; R10 is selected from H and C1-C6-alkyl optionally substituted by one or more R11; said one or more R11 is independently selected from Cl, F and hydroxy; R12, R13, R14, R15 and R16 are independently selected from H, C1-C6-alkyl optionally substituted by one or more R11, C3-C6-cycloalkyl optionally substituted by one or more R11, -SO2-C1-C6-alkyl optionally substituted by one or more R11, or wherein said R12 and R13 together with the nitrogen to which they are attached form a heterocycle optionally substituted by one or more R17; said one or more R17 is independently selected from halogen, hydroxy, NO2, CN, -N(R12)(R13), -C(O)-R16, -C(O)-OR16, -Cn-alkyl-OR16 with n=0-3, C1-C6-alkyl optionally substituted by one or more R11, and C1-C6-alkoxy optionally substituted by one or more R11; R18 is selected from -N(R12)(R13), -OR10, -C(O)-R16, -C(O)-OR16, -C(O)- N(R12)(R13), CN, and a heterocycle optionally substituted by one or more R17; and wherein at least one of R1, R2, R4, R5, R6, R7, R8 or R9 is not H; and pharmaceutically acceptable salts, stereoisomers, enantiomers, tautomers of the compounds of formula (I) as well as pharmaceutical compositions thereof and their uses in methods of reducing the virulence of bacteria that express AgrA, in methods for preventing or treating diseases caused or exacerbated by bacteria, preferably by Staphylococcus aureus, such as skin or lung infections or atopic dermatitis.本发明涉及一种化合物,其符合以下式(I),其中R1和R5分别选自H、卤素、羟基、NO2、CN、C1-C6烷基(可选地由一个或多个R11取代)、C1-C6烷氧基(可选地由一个或多个R11取代)、C3-C6环烷基(可选地由一个或多个R11取代)、-Cn-烷基-N(R12)(R13)(n=0-3)、-Cn-烷基-C(O)N(R12)(R13)(n=0-3)、-SO2-N(R14)-C(O)-R15;-Cn-烷基-N(R14)-C(O)-R15(n=0-3)、-Cn-烷基-C(O)-OR16(n=0-3)、-O(C1-C3-烷基-O)m-C1-C3-烷基-OR10(m=0-3)、-Cn-烷基-OR16(n=0-3)、-NH-Cn-烷基-R18(n=0-3);-O-Cn-烷基-R18(n=0-3);-OPO(OR10)2、-PO(OR10)2,以及可选地由一个或多个R17取代的杂环;R3选自卤素、羟基、 、CN、C1-C6烷基(可选地由一个或多个R11取代)、C1-C6烷氧基(可选地由一个或多个R11取代)、C3-C6环烷基(可选地由一个或多个R11取代)、-Cn-烷基-N(R12)(R13)(n=0-3)、-Cn-烷基-C(O)N(R12)(R13)(n=0-3)、-SO2-N(R14)-C(O)-R15;-Cn-烷基-N(R14)-C(O)-R15(n=0-3)、-Cn-烷基-C(O)-OR16(n=0-3)、-O(C1-C3-烷基-O)m-C1-C3-烷基-OR10(m=0-3)、-Cn-烷基-OR16(n=0-3)、-NH-Cn-烷基-R18(n=0-3);-O-Cn-烷基-R18(n=0-3);-OPO(OR10)2、-PO(OR10)2,以及可选地由一个或多个R17取代的杂环;R2和R4分别选自H、卤素、C1-C6烷基(可选地由一个或多个R11取代);R6、R7、R8和R9分别选自H、卤素、羟基、 、CN、C1-C6烷基(可选地由一个或多个R11取代)、C1-C6烷氧基(可选地由一个或多个R11取代)、C3-C6环烷基(可选地由一个或多个R11取代)、-Cn-烷基-N(R12)(R13)(n=0-3)、-Cn-烷基-C(O)N(R12)(R13)(n=0-3)、-SO2-N(R12)(R13)、-SO2-N(R14)-C(O)-R15;-Cn-烷基-N(R14)-C(O)-R15(n=0-3)、-Cn-烷基-C(O)-OR16(n=0-3)、-O(C1-C3-烷基-O)m-C1-C3-烷基-OR10(m=0-3)、-Cn-烷基-OR16(n=0-3)、-NH-Cn-烷基-R18(n=0-3);-O-Cn-烷基-R18(n=0-3);-OPO(OR10)2、-PO(OR10)2,以及可选地由一个或多个R17取代的杂环;R10选自H和C1-C6烷基(可选地由一个或多个R11取代);所述的一个或多个R11分别选自Cl、F和羟基;R12、R13、R14、R15和R16分别选自H、C1-C6烷基(可选地由一个或多个R11取代)、C3-C6环烷基(可选地由一个或多个R11取代)、-SO2-C1-C6烷基(可选地由一个或多个R11取代),或其中所述的R12和R13与它们连接的氮一起形成一个可选地由一个或多个R17取代的杂环;所述的一个或多个R17分别选自卤素、羟基、 、CN、-N(R12)(R13)、-C(O)-R16、-C(O)-OR16、-Cn-烷基-OR16(n=0-3)、C1-C6烷基(可选地由一个或多个R11取代)和C1-C6烷氧基(可选地由一个或多个R11取代);R18选自-N(R12)(R13)、-OR10、-C(O)-R16、-C(O)-OR16、-C(O)-N(R12)(R13)、CN,以及可选地由一个或多个R17取代的杂环;其中R1、R2、R4、R5、R6、R7、R8或R9中至少有一个不是H;以及所述的化合物的药学上可接受的盐、立体异构体、对映异构体、互变异构体,以及其在减少表达AgrA的细菌的毒力的方法中的药物组合物及其用途,用于预防或治疗由细菌引起或加重的疾病,优选由金黄色葡萄球菌引起的疾病,如皮肤或肺部感染或特应性皮炎。
-
ESR Studies of Dibenzenesulfenamidyl Radicals作者:Yozo Miura、Noboru Makita、Masayoshi KinoshitaDOI:10.1246/bcsj.50.482日期:1977.2Dibenzenesulfenamidyl radicals (2) were generated by the oxidation of dibenzenesulfenamides (1), and their ESR and visible spectra were measured. The ESR spectra were split into a 1 : 1 : 1 triplet by the interaction with the nitrogen nucleus (aN=11.26–11.49 G); in some spectra, each of the triplet was further split by the interaction with the ring protons (ao−H=ap−H=0.48–0.70, am−H=0.18–0.22 G). The二苯次磺酰胺 (1) 氧化生成二苯次磺酰胺自由基 (2),并测量其 ESR 和可见光谱。通过与氮核的相互作用,ESR 光谱被分成 1 : 1 : 1 三重峰 (aN=11.26–11.49 G);在一些光谱中,每个三重态都通过与环质子的相互作用进一步分裂(ao-H=ap-H=0.48-0.70,am-H=0.18-0.22 G)。g 值位于 2.0080–2.0083 的范围内。从结果可以得出结论,未成对电子主要分布在氮和两个硫原子上。2 衰变的动力学研究表明, 2 以二级动力学衰变并且对大气氧不敏感。
表征谱图
-
氢谱1HNMR
-
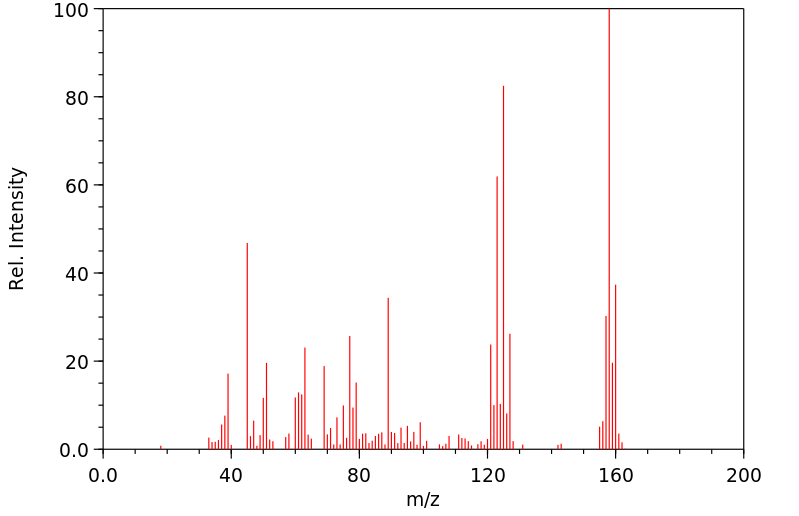
质谱MS
-
碳谱13CNMR
-
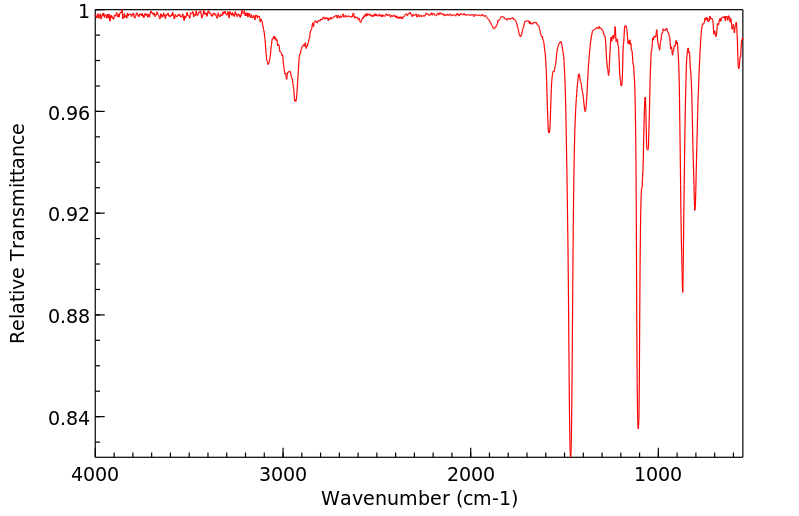
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
邻氯苯硫酚
邻巯基苯乙酮肟
苯硫醇,4-氨基-2,5-二氟-
苯硫醇,2-[(丙基硫代)甲基]-
苯硫醇,2-(氨基甲基)-6-氟-
苯硫醇
苯硫酚钾
苯硫酚钠
苯硫酚
苯六硫酚
甲苯-3,4-二硫酚
烯丙基(邻巯基苯基)甲基硫醚
戊甲基苯硫醇
对氟苯硫酚
对叔丁基硫酚
对-(三甲基甲硅烷)苯硫酚
四巯基苯
五氯苯硫酚锌盐
五氯苯硫酚
五氟苯硫酚
三(巯基苯基)(甲基)硅烷
S-(2-溴-2-氯-1,1-二氟乙基)半胱氨酸
6-氨基-2-氟-3-甲基苯硫醇
6-氨基-2,3-二氟苯硫醇
5-溴-1,3-苯基二硫醇
5-氯-2-甲基苯硫酚
5-氯-2-(甲硫基)苯硫酚
5-氨基-2-氯-4-氟苯硫醇
5-氟-2-甲氧基苯硫醇
5-氟-2-甲基硫代苯酚
5-氟-2-巯基苄醇
4H-吡喃-4-酮,2,3-二氢-2-甲基-,(2R)-(9CI)
4-辛氧基苯硫醇
4-羟基苯硫醇钠
4-羟基苯硫酚
4-羟-3-甲基苯硫酚
4-碘代苯-1-硫醇
4-甲苯硫酚
4-甲硫基苯硫醇
4-甲氧基苯硫酚
4-甲氧基-3-<(2-甲氧基吡啶-5-基)甲基>苯硫酚
4-甲氧基-2-硫基苯甲醛
4-甲氧基-2-甲基硫代苯酚
4-甲基苯硫醇铅
4-甲基磺酰氧基苯硫酚
4-甲基-2-硫基苯甲醛
4-甲基-2,3,5,6-四氟苯硫酚
4-环戊基苯硫醇
4-环己基-苯硫酚
4-环丙基苯硫醇