4-phenyl-1,2,3-selenadiazole | 25660-64-4
中文名称
——
中文别名
——
英文名称
4-phenyl-1,2,3-selenadiazole
英文别名
4-phenyl-[1,2,3]-selenadiazole;4-phenyl-[1,2,3]selenadiazole;4-phenylselenadiazole
CAS
25660-64-4
化学式
C8H6N2Se
mdl
——
分子量
209.109
InChiKey
LEWDFWSHOZUNRZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:227.23°C (estimate)
计算性质
-
辛醇/水分配系数(LogP):1.2
-
重原子数:11
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:25.8
-
氢给体数:0
-
氢受体数:2
SDS
反应信息
-
作为反应物:描述:4-phenyl-1,2,3-selenadiazole 在 丙烯酸乙酯 作用下, 反应 15.0h, 以80%的产率得到苯乙炔参考文献:名称:1,2,3-硒代二唑与烯烃的反应摘要:当在130°C下用过量的烯烃处理由环状酮合成的1,2,3-硒代二唑时,会添加乙烯基,该乙烯基是通过1,2,3-硒代二唑进行脱氮而原位生成的。碳-碳双键随后分子内环化有效地进行,从而以中等至良好的收率得到了相应的二氢硒代苯烯,并形成了相应的副产物1,4-二硒代和硒代苯。在该反应中,在1,2,3-硒代二唑的合成中用作起始原料的酮的环上的碳原子数对产物的选择性起着重要作用。与由环状酮制得的1,2,3-硒代二唑的反应相反,在1,2的反应中,DOI:10.1016/s0022-328x(00)00484-8
-
作为产物:描述:参考文献:名称:某些芳基1,2,3-三硒二唑的合成及1 H核磁共振谱摘要:制备了一系列芳基1,2,3-3-硒代二唑,并记录了它们的核磁共振光谱参数。由于4-芳基取代基中的间位或对位取代而引起的5-质子的化学位移范围与取代苯侧链中相应质子的化学位移范围相似。DOI:10.1039/p19740000030
-
作为试剂:描述:参考文献:名称:Reaction of 1,2,3-Selenadiazoles with Phosphines摘要:DOI:10.1023/b:cohc.0000033546.79059.91
文献信息
-
Synthesis, characterization, antiamoebic activity and cytotoxicity of novel 2-(quinolin-8-yloxy) acetohydrazones and their cyclized products (1,2,3-thiadiazole and 1,2,3-selenadiazole derivatives)作者:Faisal Hayat、Attar Salahuddin、Jamil Zargan、Amir AzamDOI:10.1016/j.ejmech.2010.09.066日期:2010.121,2,3-selenadiazole derivatives were synthesized by the cyclization of novel 2-(quinolin-8-yloxy) acetohydrazones. In vitro antiamoebic activity was performed against HM1: IMSS strain of Entamoeba histolytica. The results showed that all the 2-(quinolin-8-yloxy) acetohydrazones were more active than their cyclized products (1,2,3-thiadiazole and 1,2,3-selenadiazole derivatives). SAR showed that the
-
Ionic Liquid as Soluble Support for Synthesis of 1,2,3-Thiadiazoles and 1,2,3-Selenadiazoles作者:Anil Kumar、Manoj Kumar Muthyala、Sunita Choudhary、Rakesh K. Tiwari、Keykavous ParangDOI:10.1021/jo301607a日期:2012.10.193-selenadiazoles was achieved using an ionic liquid as a novel soluble support. Ionic liquid-supported sulfonyl hydrazine was synthesized and reacted with a number of ketones to afford the corresponding ionic liquid-supported hydrazones that were converted to 1,2,3-thiadiazoles in the presence of thionyl chloride. The reaction of ionic liquid-supported hydrazones with selenium dioxide in acetonitrile afforded
-
Transition metal heterocyclic chemistry作者:Armin J. Mayr、Benjamin Carrasco-Flores、Francisco Cervantes-Lee、Keith H. Pannell、László Párkányi、Krishan RaghuVeerDOI:10.1016/0022-328x(91)86290-7日期:1991.3Reactions of substituted 1,2,3-selena- and thia-diazoles with (η5-C5H4R)Mn(CO)2THF, R H, Me, lead to isolable blue complexes in which THF is replaced by the diazole ligand. The X-ray structure of the benzothiadiazole complex is reported and shows the intact diazole bonded via N2 to manganese. Ab initio calculations on 1,2,3-thiadiazole generated a structure and protonation energies that confirm N2
-
Tributylstannyl Radical-Catalyzed Reaction of 1,2,3-Selenadiazoles with Olefins or Dienes作者:Yutaka Nishiyama、Yasunobu Hada、Masahiro Anjiki、Kazuya Miyake、Sakiko Hanita、Noboru SonodaDOI:10.1021/jo010893t日期:2002.3.1It was found that the reaction of 1,2,3-selenadiazoles derived from cyclic ketones with olefins or dienes was markedly promoted by a catalytic amount of tributylstannyl radical, which was generated in situ from tributylstannyl hydride or allyltributylstannane and AIBN, to give the corresponding dihydroselenophenes in moderate to good yields. In contrast, when 1,2,3-selenadiazoles prepared from linear
-
Synthesis and characterisation of cyclopentadienylcobalt and pentamethylcyclopentadienylcobalt diselenolenes作者:Christopher P. Morley、Richard R. VaughanDOI:10.1039/dt9930000703日期:——Reaction of [Co(C5R15)(CO)2] (R1 = H or Me) with 1,2,3-selenadiazoles in toluene under reflux in the presence of an excess of elemental selenium leads in moderate yield to the diselenolenes [Co(C5R15)SeC(R2)=C(R3)Se}] [R2-R3=(CH2)6, CH=CH(CH2), or CH=CH(CH2)2CH=CH; R2=Ph, R3=H; R2=R3=Ph]. In particular this procedure provides access to novel compounds without electron-withdrawing substituents. The products have been characterised by H-1 NMR, IR, UV/VIS and mass spectroscopy, microanalysis and cyclic voltammetry. Each diselenolene undergoes a fully reversible one-electron reduction, and exhibits a low-energy electronic transition at ca. 800 nm. Of the substituents (R1,R2,R3), R1 has the greatest influence on the spectroscopic and electrochemical properties. For structurally similar compounds there is a linear correlation between the half-wave potential for the reduction and the energy of the first electronic absorption band.
表征谱图
-
氢谱1HNMR
-
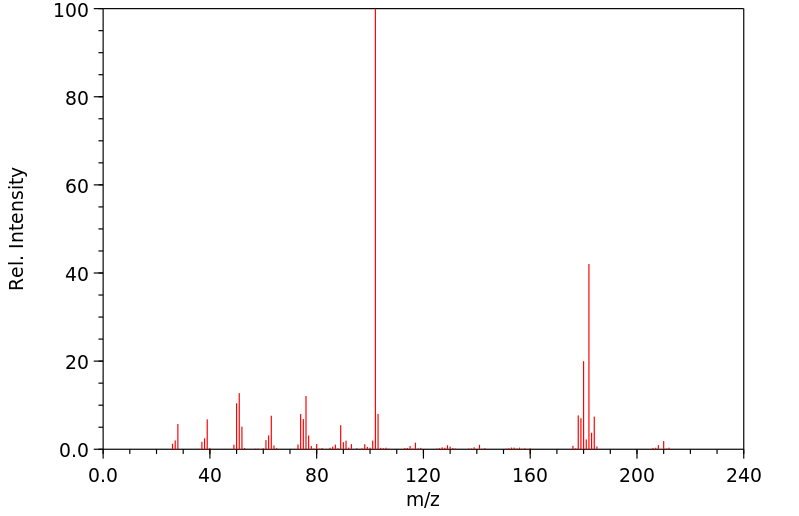
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫