4,4'-二异丙基联苯 | 18970-30-4
中文名称
4,4'-二异丙基联苯
中文别名
4,4’-二异丙基联苯
英文名称
4,4' diisopropylbiphenyl
英文别名
4,4'-Diisopropyl-diphenyl;4,4'-DIISOPROPYLBIPHENYLE;4,4'-Diisopropylbiphenyl;1-propan-2-yl-4-(4-propan-2-ylphenyl)benzene
CAS
18970-30-4
化学式
C18H22
mdl
——
分子量
238.373
InChiKey
NUEUMFZLNOCRCQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:66°C
-
沸点:335 °C
-
密度:0.935±0.06 g/cm3(Predicted)
-
保留指数:1963
-
稳定性/保质期:
在常温常压下稳定,避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):6.6
-
重原子数:18
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.333
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36/37
-
危险类别码:R36
-
WGK Germany:3
-
海关编码:2902909090
-
储存条件:常温下应密闭避光保存,并置于通风干燥处。
SDS
4,4'-二异丙基联苯 修改号码:2
模块 1. 化学品
产品名称: 4,4'-Diisopropylbiphenyl
修改号码: 2
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 4,4'-二异丙基联苯
百分比: ....
CAS编码: 18970-30-4
分子式: C18H22
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
4,4'-二异丙基联苯 修改号码:2
模块 5. 消防措施
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色类白色
气味: 无资料
pH: 无数据资料
熔点: 66°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度: 无资料
模块 10. 稳定性和反应性
稳定性: 一般情况下稳定。
反应性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
4,4'-二异丙基联苯 修改号码:2
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布): 针对危险化学品的安全使用、生产、储存、运输、装
卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 4,4'-Diisopropylbiphenyl
修改号码: 2
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 4,4'-二异丙基联苯
百分比: ....
CAS编码: 18970-30-4
分子式: C18H22
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
4,4'-二异丙基联苯 修改号码:2
模块 5. 消防措施
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色类白色
气味: 无资料
pH: 无数据资料
熔点: 66°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度: 无资料
模块 10. 稳定性和反应性
稳定性: 一般情况下稳定。
反应性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
4,4'-二异丙基联苯 修改号码:2
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布): 针对危险化学品的安全使用、生产、储存、运输、装
卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3,4-二异丙基联苯 3,4'-di-isopropylbiphenyl 61434-46-6 C18H22 238.373 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1-(4'-(1-methylethyl)biphenyl-4-yl)ethanone 102714-30-7 C17H18O 238.329 —— 4,4'-bis(2-hydroxy-2-propyl)biphenyl 22726-67-6 C18H22O2 270.371 —— 4-(1-hydroxy-1-methylethyl)-4'-(1-methylethyl)biphenyl 141987-55-5 C18H22O 254.372 3,4-二异丙基联苯 3,4'-di-isopropylbiphenyl 61434-46-6 C18H22 238.373 —— 4,4'-bis-(α-chloro-isopropyl)-biphenyl 87188-59-8 C18H20Cl2 307.263 —— 4-(4-isopropylphenyl)benzoic acid 5728-35-8 C16H16O2 240.302 —— 4,4'-diisopropenylbiphenyl 22726-68-7 C18H18 234.341 —— 4-(1-hydroperoxy-1-methylethyl)-4'-(1-methylethyl)biphenyl 120540-29-6 C18H22O2 270.371
反应信息
-
作为反应物:描述:4,4'-二异丙基联苯 在 N-羟基邻苯二甲酰亚胺 、 硫酸 、 双氧水 、 氧气 、 cobalt(II) diacetate tetrahydrate 作用下, 以 乙腈 为溶剂, 反应 24.0h, 生成 4,4'-二羟基联苯参考文献:名称:过渡金属盐和 N-羟基邻苯二甲酰亚胺催化的烷基和酰基芳烃自由基有氧氧化中的溶剂和温度效应:用于液晶和交联聚合物合成对羟基苯甲酸、二酚和二烯的新工艺摘要:在 N-羟基邻苯二甲酰亚胺和 Co(II) 盐的催化下,4,4'-二异丙基联苯和 2,6-二异丙基萘的有氧氧化得到相应的叔苄醇,在温和条件下(温度 30-60°)具有高转化率和选择性C 和大气压)。由于 C. Walling 的开创性工作以及最近 KU Ingold 及其同事在定量动力学基础上的结论性解决方案,溶剂和温度效应强烈影响有氧氧化的选择性。这与生成苯乙酮衍生物的烷氧基的 β 断裂速率与生成叔苯甲醇的氢原子夺取速率之间的比率有关。这些后者被有效地转化为二酚以生产液晶,通过与 H2O2 反应,或通过脱水反应生成可用作交联剂的二烯。Mn(NO3)2和Co(NO3)2催化对羟基苯乙酮有氧氧化...DOI:10.1021/op034137w
-
作为产物:描述:参考文献:名称:有机电极过程中的自由基反应-III:铂阳极上的芳烃在含高氯酸盐支持电解质的乙腈溶液中的反应摘要:某些高氯酸盐在乙腈溶液中的电解都是在不存在和存在芳族烃(例如甲苯和枯烯)的情况下进行的。形成的产物表明该反应涉及高氯酸根离子(或自由基)对烃的氧化。检测到烃的无氧偶联产物,但是这些反应的程度通常很小。讨论了形成这些产物的可能的反应路径。DOI:10.1016/0040-4020(67)85131-7
文献信息
-
Copper quinolate: A simple and efficient catalytic complex for coupling reactions作者:Fengtian Wu、Huiqin Li、Jianwei XieDOI:10.1002/aoc.5303日期:2020.1quinolate‐catalyzed N‐arylation of aryl halides and imidazoles. A wide range of products were obtained in moderate to excellent yields under the optimal reaction conditions. Applying standard conditions, the model reaction could be performed on a gram scale. This method also presents a new avenue to the “click” reaction of terminal alkynes, benzyl bromide, and sodium azide and to the construction of C–C
-
Shape-Selective Isopropylation of Aromatic Hydrocarbons over H-Mordenite in Supercritical Carbon Dioxide Medium作者:Subhash Chandra Laha、Hiroaki Naiki、Kenichi Komura、Yoshihiro SugiDOI:10.1246/bcsj.20110214日期:2011.11.15The isopropylation of aromatic hydrocarbons isobutylbenzene (IBB), naphthalene (NP), and biphenyl (BP) was examined over H-mordenite (MOR), H-β (BEA), and H-Y (FAU) zeolites in supercritical carbon dioxide (sc-CO2) medium. MOR was only selective for the formation of the least bulky 4-isobutylcumene (4-IBC) in the isopropylation of IBB. In particular, the catalytic activity and selectivity for 4-IBC were enhanced by the dealumination of MOR; MOR with 110 of SiO2/Al2O3 ratio rendered the highest performance; however, the catalytic activity was decreased by further dealumination. Thermogravimetric analyses confirmed the reduction of coke formation on the catalysts in sc-CO2 medium, preventing the deactivation of MOR. Shape-selective formation of the least bulky isomers, 2,6-diisopropylnaphthalene (2,6-DIPN) and 4,4′-diisopropylbiphenyl (4,4′-DIPB), was also observed in the isopropylation of NP and BP over MOR in sc-CO2. sc-CO2 works as an efficient medium to access and/or replace substrates and their products to/from acidic sites in the MOR channels. In particular, the removal of coke precursors from acidic sites on the zeolite is enhanced by the sc-CO2 medium, resulting in decreased coke formation.芳烃异丙基化反应中,异丁基苯(IBB)、萘(NP)和联苯(BP)在超临界二氧化碳(sc-CO2)介质中进行了对H-莫来石(MOR)、H-β(BEA)和H-Y(FAU)沸石的研究。MOR在对IBB的异丙基化反应中仅对形成体积最小的4-异丁基倍氯烷(4-IBC)具有选择性。特别地,通过去铝化处理MOR可以增强其对4-IBC的催化活性和选择性;具有110 SiO2/Al2O3比的MOR表现出最高的性能;然而,进一步的去铝化会降低催化活性。热重分析证实,在sc- 介质中催化剂上的焦炭形成减少,从而防止了MOR的失活。在对NP和BP的异丙基化反应中,也观察到在MOR上形状选择性形成体积最小的异构体2,6-二异丙基萘(2,6-DIPN)和4,4'-二异丙基联苯(4,4'-DIPB)。sc- 作为一种高效介质,可以访问和/或替代MOR通道中的酸性位点的底物及其产物。特别是,sc- 介质增强了从沸石酸性位点去除焦炭前驱体的能力,从而减少了焦炭的形成。
-
Equilibria of isomeric transformations of alkylbiphenyls作者:I.Yu Roshchupkina、T.N Nesterova、A.M RozhnovDOI:10.1016/0021-9614(87)90137-6日期:1987.3Abstract Equilibria of mutual transformations of mono-, di-, and tri-alkylbiphenyls ( abp ) were investigated in the liquid phase at 308 to 423 K. On the basis of experimental equilibrium constants, values of ΔrHmo/(kJ·mol−1) and ΔrSmo/(J·K−1·mol−1) were calculated. Below are given correspondingly: reaction, compound and values for Et- bp (I), i-Pr- bp (II), and t-Bu- bp (III): 4- abp = 3- abp , I摘要 研究了液相中单、二和三烷基联苯 ( abp ) 相互转化的平衡,温度为 308 至 423 K。根据实验平衡常数,ΔrHmo/(kJ·mol−1) 值和ΔrSmo/(J·K-1·mol-1)被计算。下面分别给出:Et-bp (I)、i-Pr-bp (II) 和 t-Bu-bp (III) 的反应、化合物和值: 4- abp = 3- abp , I, 0.23, 5.76 ; 二、(0.45±0.41)、(5.72±1.13);三、(0.48±0.53)、(4.83±0.53);2- abp = 4- abp , I, -3.3, -5.76; II、-12.6、-5.76;III、-15.4、-5.76;3,5-di-abp = 3,3'-di-abp, I, -0.1, 5.76; 二、(0±0.60)、(5.98±1.65);三、(-1.34±0.67)、(4.48±1.87);3
-
Aerobic oxidation of alkylaromatic hydrocarbons to hydroperoxides catalysed by N-hydroxyimides in ionic liquids as solvents作者:Gabriela Dobras、Beata OrlińskaDOI:10.1016/j.apcata.2018.05.012日期:2018.7alkylaromatic hydrocarbons to hydroperoxides with oxygen in the presence of N-hydroxyimides (N-hydroxyphthalimide, N-hydroxysuccinimide, 4-dodecyloxycarbonyl-N-hydroxyphthalimide, N,N-dihydroxypyromellitimide, N-acetoxyphthalimide) in ionic liquids as solvents. Cumene, ethylbenzene, mono- and di-isopropyl derivatives of naphthalene and biphenyl were used as starting materials. As solvents, ionic liquids composed本文报道了在N-羟基酰亚胺(N-羟基邻苯二甲酰亚胺,N-羟基琥珀酰亚胺,4-十二烷氧基羰基-N-羟基邻苯二甲酰亚胺,N,N-二羟基邻苯二甲酰亚胺,N-乙酰氧基邻苯二甲酰亚胺)存在下用氧将烷基芳烃氧化为氢过氧化物的研究。液体作为溶剂。枯烯,乙苯,萘和联苯的一异丙基和二异丙基衍生物用作起始原料。作为溶剂,由具有乙基,丁基,己基或辛基取代基的1-烷基-3-甲基咪唑阳离子和[BF 4 ],[PF 6 ],[NTf 2 ],[CF 3等阴离子组成的离子液体施加了SO 3,[OcOSO 3 ]和[CH 3 OSO 3 ]。已经确定有机溶剂可以被离子液体代替,其不会加速氢过氧化物的分解,并且具有相对较高的氧气和催化剂溶解度以及低粘度的特征。在研究的IL中,这些具有[NTf 2 ]和[PF 6 ]阴离子的离子满足上述要求。
-
Lanthanoid Exchanged Mordenites as Catalysts for the Isopropylation of Biphenyl作者:Yoshihiro Sugi、Seiji Watanabe、Hiroaki Naiki、Ken-ichi Komura、Yoshihiro KubotaDOI:10.1246/bcsj.20110022日期:2011.6.15Liquid-phase isopropylation of biphenyl (BP) with propene was studied to understand the acidities resulting from lanthanoid exchanged sodium mordenite (Ln,NaMOR; Ln: La, Ce, Pr, Sm, Dy, and Yb). The acidities of La3+ exchanged sodium mordenite (La,NaMOR) appeared at around 0.6–0.8 exchange calculated from La/3Al molar ratio. Similar acidities appeared with all Ln,NaMORs. The resultant zeolites have Brønsted acidic sites appearing near unsaturated lanthanoid cations. The isopropylation of BP predominantly afforded 4,4′-diisopropylbiphenyl (4,4′-DIPB) among DIPB isomers over all Ln,NaMORs. These catalyses occur on the acidic sites in the channels. The exchanged catalysts had high selectivities for 4,4′-DIPB even at temperatures as high as 300 °C although the selectivities decreased by the isomerization of 4,4′-DIPB over H-mordenite (HMOR) with similar SiO2/Al2O3 ratio. These results indicate the external acid sites are lowly active for the isopropylation of BP and the isomerization of 4,4′-DIPB. The combustion of coke-deposits on Ln,NaMORs, particularly Ce,NaMOR, used for the isopropylation occurred at lower temperatures than that on HMOR because the lanthanoids dispersed in MOR channels work as an oxidation catalyst.为了了解镧系元素交换莫代森钠(Ln,NaMOR;Ln:La、Ce、Pr、Sm、Dy 和 Yb)产生的酸性,对联苯(BP)与丙烯的液相异丙基化进行了研究。根据 La/3Al 摩尔比计算,La3+ 交换莫来石钠(La,NaMOR)的酸度出现在 0.6-0.8 左右。所有 Ln,NaMOR 的酸度都相似。由此产生的沸石具有布氏酸性位点,出现在不饱和镧系阳离子附近。在所有 Ln、NaMORs 上,BP 的异丙基化反应主要产生 DIPB 异构体中的 4,4′-二异丙基联苯(4,4′-DIPB)。这些催化作用发生在通道中的酸性位点上。交换催化剂对 4,4′-DIPB 的选择性很高,即使在高达 300 °C 的温度下也是如此,尽管在具有相似 SiO2/Al2O3 比率的 H-莫代石(HMOR)上异构化 4,4′-DIPB 时选择性会降低。这些结果表明,外部酸性位点对 BP 的异丙基化和 4,4′-DIPB 的异构化活性较低。用于异丙基化的 Ln、NaMOR(尤其是 Ce、NaMOR)上的焦炭沉积物的燃烧温度低于 HMOR,这是因为分散在 MOR 通道中的镧系元素起到了氧化催化剂的作用。
表征谱图
-
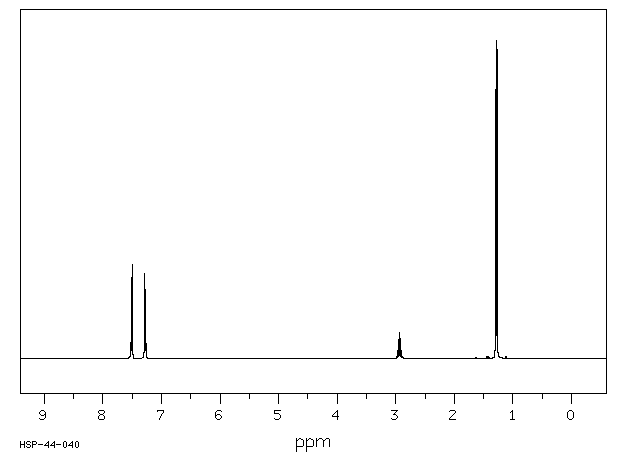
氢谱1HNMR
-
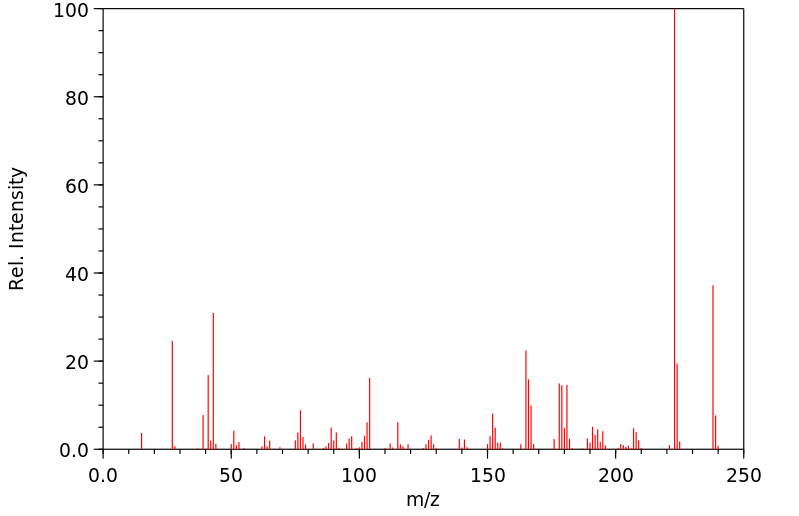
质谱MS
-
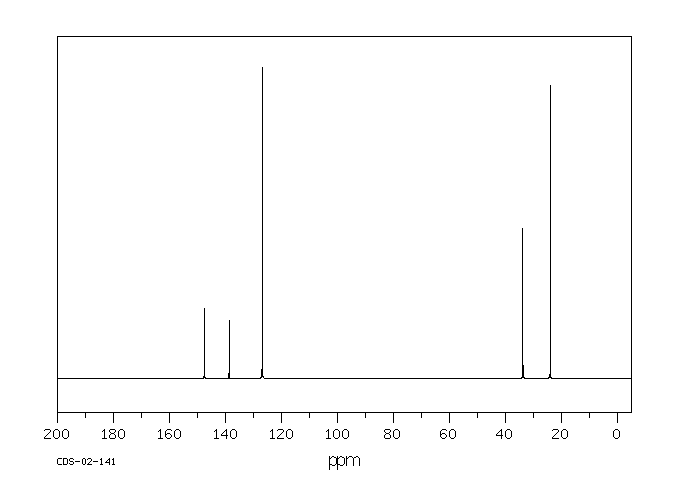
碳谱13CNMR
-
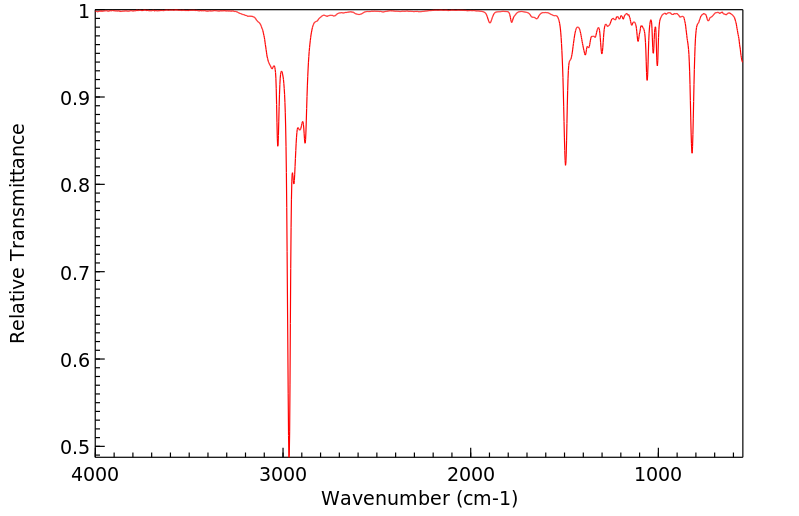
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫