4-氯苯甲酸丙酯 | 25800-30-0
中文名称
4-氯苯甲酸丙酯
中文别名
——
英文名称
propyl 4-chlorobenzoate
英文别名
——
CAS
25800-30-0
化学式
C10H11ClO2
mdl
MFCD12178053
分子量
198.649
InChiKey
BLEFFSGNRQPNCA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
保留指数:1406;1415;1420;1419;1428;1434
计算性质
-
辛醇/水分配系数(LogP):4
-
重原子数:13
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
SDS
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Selective Cross-Coupling of (Hetero)aryl Halides with Ammonia To Produce Primary Arylamines using Pd-NHC Complexes摘要:Herein we report the first example of (hetero)-arylation of ammonia using a monoligated palladium-NHC complex. The new, rationally designed, precatalyst (DiMeIHept(Cl))Pd(allyl)Cl featuring highly branched alkyl chains has been shown to be effective in selective aminations across a range of challenging substrates, including nitrogen containing heterocycles and those featuring base-sensitive functionality. The less bulky Pd-PEPPSI-IPent(Cl) precatalyst performs well for ortho-substituted aryl halides, giving monoarylated products in high yield with good selectivity.DOI:10.1021/acs.organomet.6b00830
-
作为产物:参考文献:名称:Some Reactions of the Trifluoromethyl Group in the Benzotrifluoride Series. II. Alcoholysis1摘要:DOI:10.1021/ja01162a029
文献信息
-
Synthesis of Esters by Functionalisation of CO2申请人:Commissariat a L'Energie Atomique et aux Energies Alternatives公开号:US20170240485A1公开(公告)日:2017-08-24The invention relates to a method for (I) producing a carboxylic ester of formula (I). Said method comprises the steps of: a) bringing an organosilane/borane of formula Si or B into contact with CO 2 , in the presence of a catalyst and an electrophilic compound of formula (III), the groups R 1 , R 2 , R 3 , R 4 , R 5 , Y, and M′ being as defined in claim 1; and optionally b) recovering the compound of formula (I) produced.
-
Facile oxidation of benzyl ethers by the 2-nitrobenzenesulfonylperoxyl intermediate generated from 2-nitrobenzenesulfonyl chloride and superoxide作者:Yong Hae Kim、Yong Il Kim、Joong Young KimDOI:10.1039/a800106e日期:——Various benzyl ethers react with a 2-nitrobenzenesulfonylperoxyl radical intermediate generated from 2-nitrobenzenesulfonyl chloride and potassium superoxide at –25 °C in acetonitrile to give the corresponding esters in high yields.
-
Facile and efficient gold-catalyzed aerobic oxidative esterification of activated alcohols作者:Lianyue Wang、Jun Li、Wen Dai、Ying Lv、Yi Zhang、Shuang GaoDOI:10.1039/c3gc42075b日期:——facile and efficient methodology is presented for the direct oxidative esterification of alcohols with alcohols catalyzed by NaAuCl4. Just in the presence of a low catalytic amount of base additive, the newly developed catalytic system proceeds with high selectivity and broad substrate scope under mild conditions with dioxygen or air as the environmentally benign terminal oxidant. Various alcohols including提出了一种简便有效的方法,将醇与NaAuCl 4催化的醇直接氧化酯化。仅在催化量低的碱添加剂存在下,新开发的催化系统在温和的条件下,以双氧或空气作为环境友好的终端氧化剂,具有高的选择性和广泛的底物范围。使各种醇(包括苄醇和烯丙基醇)与甲醇,甚至与长链脂族醇平稳反应,以良好至极佳的收率(高达95%的收率)提供所需的产物。本系统显示出高催化活性,TOF高达219 h -1。反应过程的动力学研究为催化途径提供了基本的见识,并基于控制实验的结果提出了可能的反应途径。进行XPS,TEM和UV-vis以表征本催化体系中Au催化剂的化学状态。结果表明,Au纳米颗粒原位生成并负载在K 2 CO 3上,形成了简单,可回收和选择性的醇直接氧化酯化催化剂体系。
-
Solvent-Free Esterification of Carboxylic Acids and Alcohols in the Presence of Silphos [PCl<sub>3-n</sub>(SiO<sub>2</sub>)<sub>n</sub>] as a Heterogeneous Phosphine Reagent作者:Ambati Narasimha Rao、Kumaran Ganesan、Chandra Kant ShindeDOI:10.1080/00397911.2011.555903日期:2012.8.1Abstract An efficient solvent-free method for the preparation of esters from various aromatic and aliphatic acids with primary, secondary, and tertiary alcohols using a heterogeneous phosphine reagent, silphos [PCl3-n(SiO2)n], in good yields is reported. GRAPHICAL ABSTRACT摘要 报道了一种使用多相膦试剂 silphos [PCl3-n(SiO2)n] 从各种芳香族和脂肪酸与伯醇、仲醇和叔醇制备酯的有效无溶剂方法,收率良好。图形概要
-
Catalytic Action of Azolium Salts. VIII. Oxidative Aroylation with Arenecarbaldehydes Catalyzed by 1,3-Dimethylbenzimidazolium Iodide.作者:Akira MIYASHITA、Yumiko SUZUKI、Izuru NAGASAKI、Chie ISHIGURO、Ken-ichi IWAMOTO、Takeo HIGASHINODOI:10.1248/cpb.45.1254日期:——Refluxing of a mixture of benzaldehyde (1a), 1, 3-dimethylbenzimidazolium iodide (7), p-nitroaniline (9b) as an oxidizing agent, and 1, 8-diazabicyclo[5, 4, 0]-7-undecene (DBU) in MeOH afforded methyl benzoate (2a) in good yield. Other methyl arenecarboxylates 2 were similarly obtained from arenecarbaldehydes 1. We showed that this aroylation proceeds via the 2-aroyl-1, 3-dimethylbenzimidazolium salt (8). The 1, 2, 4-triazolium salt (18) and the naphtho[1, 2-d]imidazolium salt (19) were also effective catalysts for this oxidative aroylation. However, the aroylation did not proceed with the imidazolium salt (20). In the presence of flavins (25a-c), arenecarbaldehydes 1 reacted in MeOH under aerobic conditions catalyzed by the benzimidazolium salt 7 to give the corresponding methyl arenecarboxylates 2. 1-Methyl-3-[3-(10-phenylisoalloxazin-3-yl)propyl]benzimidazolium bromide (27) is an effective complex catalyst for this oxidative aroylation.将苯甲醛(1a)、1,3-二甲基苯并咪唑鎓碘化物(7)和作为氧化剂的4-硝基苯胺(9b)与1,8-二氮杂双环[5,4,0]十一碳-7-烯(DBU)的甲醇溶液回流反应,可得到产率良好的苯甲酸甲酯(2a)。其他甲基芳香羧酸酯2也可通过类似的途径从芳香醛1得到。我们证明,这种羰基化反应是通过2-羰基-1,3-二甲基苯并咪唑鎓盐(8)进行的。1,2,4-三唑鎓盐(18)和萘并[1,2-d]咪唑鎓盐(19)也是这种氧化羰基化反应的有效催化剂。然而,咪唑鎓盐(20)并不能促使该反应进行。在黄素(25a-c)存在下,芳香醛1在甲醇中经苯并咪唑鎓盐7催化的有氧条件下反应,生成相应的甲基芳香羧酸酯2。1-甲基-3-[3-(10-苯基异黄酮-3-基)丙基]苯并咪唑鎓溴化物(27)是一种有效的氧化羰基化反应复合催化剂。
表征谱图
-
氢谱1HNMR
-
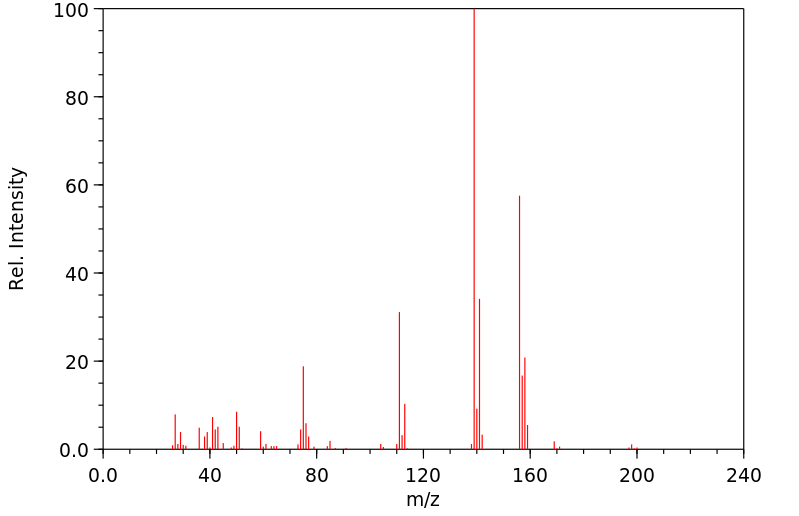
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫