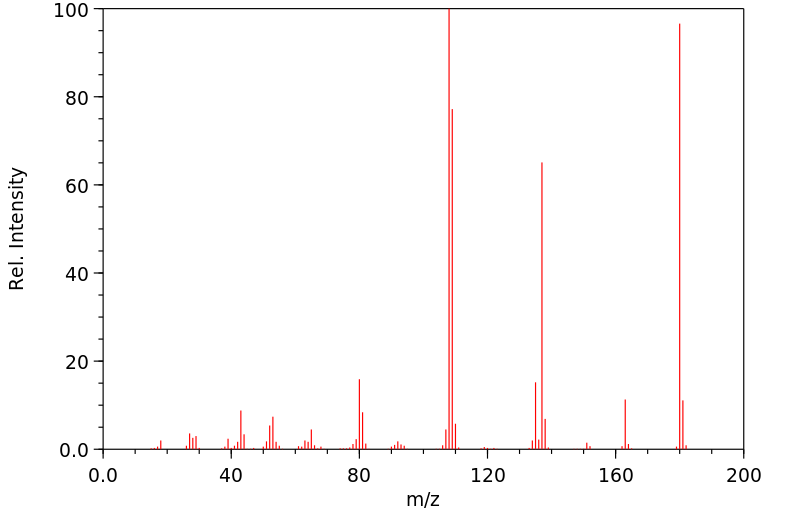
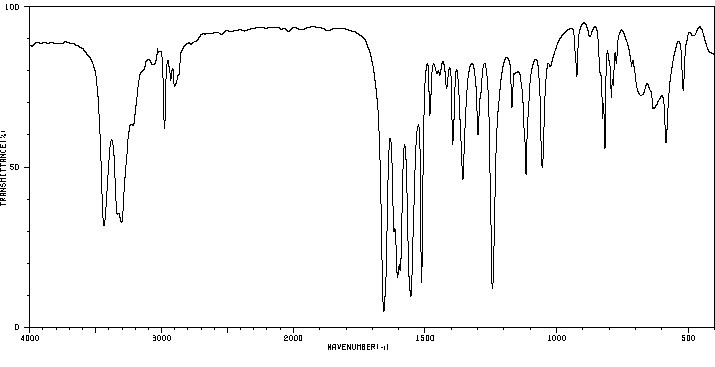
代谢
/Dulcin (DL)/ 在兔子口服给药后以尿苷酸-N-葡萄糖苷酸形式排出。... 已经从兔肝中鉴定出七种 /UDP-葡萄糖苷酸转移酶 (UGT)/ 同工酶(UGT1A3、UGT1A4、UGT1A6、UGT1A7、UGT2B13、UGT2B14 和 UGT2B16),但这些 UGT 以 DL 作为底物尚未被研究。在这项工作中,通过使用兔肝微粒体(RabLM)和克隆/表达的兔 UGT 同工酶,研究了催化 DL 葡萄糖苷酸形成的 UGT 同工酶的鉴定。通过使用电喷雾液相色谱-串联质谱法,定量测定了 RabLM 和表达每个 UGT 同工酶的 COS-7 细胞匀浆中 DL-N-葡萄糖苷酸(DNG)的产生。使用 Eadie-Hofstee 图分析 RabLM 中 DNG 形成得到的 Vmax 为 0.911 nM/min/mg 蛋白质,Km 为 1.66 mM。DNG 形成仅由克隆表达的兔 UGT1A7 和 UGT2B16 催化(Vmax 分别为 3.98 和 1.16 pmol/min/mg 蛋白质,Km 分别为 1.23 和 1.69 mM)。辛基没食子酸通过抑制 UGT1A7 证实了 UGT1A7 对 DNG 形成的重要贡献。进一步研究表明辛基没食子酸通过竞争性抑制 RabLM 中的 DNG 生产(Ki = 0.149 mM)。这些结果表明 UGT1A7 是在 RabLM 中催化 DL 的 N-葡萄糖苷酸化的主要同工酶。
... /Dulcin (DL)/ is excreted as a urinary ureido-N-glucuronide after oral administration to rabbits. ... Seven /UDP-glucuronosyltransferase (UGT)/ isoforms (UGT1A3, UGT1A4, UGT1A6, UGT1A7, UGT2B13, UGT2B14, and UGT2B16) have been identified from rabbit liver, but these UGTs have not been investigated using DL as a substrate. In this work, the identities of UGT isoforms catalyzing the formation of DL glucuronide were investigated using rabbit liver microsomes (RabLM) and cloned/expressed as rabbit UGT isoforms. DL-N-glucuronide (DNG) production was determined quantitatively in RabLM and homogenates of COS-7 cells expressing each UGT isoform by using electrospray liquid chromatography-tandem mass spectrometry. Analysis of DNG formation using RabLM, by Eadie-Hofstee plot, gave a Vmax of 0.911 nM/min/mg protein and the Km of 1.66 mM. DNG formation was catalyzed only by cloned expressed rabbit UGT1A7 and UGT2B16 (Vmax of 3.98 and 1.16 pmol/min/mg protein and a Km of 1.23 and 1.69 mM, respectively). Substrate inhibition of UGT1A7 by octylgallate confirmed the significant contribution of UGT1A7 to the formation of DNG. Octylgallate was further shown to competitively inhibit DNG production by RabLM (Ki = 0.149 mM). These results demonstrate that UGT1A7 is the major isoform catalyzing the N-glucuronidation of DL in RabLM.
来源:Hazardous Substances Data Bank (HSDB)