乙酸山嵛基酯 | 822-26-4
中文名称
乙酸山嵛基酯
中文别名
——
英文名称
1-docosanol acetate
英文别名
1-docosyl acetate;docosyl acetate;acetic acid docosyl ester;Essigsaeure-docosylester;1-docosanol, acetate;Docosanolyl-1-acetat
CAS
822-26-4
化学式
C24H48O2
mdl
——
分子量
368.644
InChiKey
AWYRDXDPCQRJHE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
保留指数:2594
计算性质
-
辛醇/水分配系数(LogP):11
-
重原子数:26
-
可旋转键数:22
-
环数:0.0
-
sp3杂化的碳原子比例:0.958
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
SDS
反应信息
-
作为产物:描述:alkaline earth salt of/the/ methylsulfuric acid 在 acetic acid ester 作用下, 生成 乙酸山嵛基酯参考文献:名称:Stoll; Rouve, Helvetica Chimica Acta, 1944, vol. 27, p. 957摘要:DOI:
文献信息
-
Zn(ClO4)2·6H2O as a Powerful Catalyst for a Practical Acylation of Alcohols with Acid Anhydrides作者:Giuseppe Bartoli、Marcella Bosco、Renato Dalpozzo、Enrico Marcantoni、Massimo Massaccesi、Letizia SambriDOI:10.1002/ejoc.200300458日期:2003.12new protocol for the acylation of alcohols with anhydrides in the presence of Zn(ClO4)2·6H2O as the catalyst is reported. The activity of Zn(ClO4)2·6H2O has been proven to be superior to that exerted by dry Mg(ClO4)2 and by metal triflates. Its efficiency allows reactions between poorly reactive substrates, such as sterically hindered tertiary alcohols and aromatic anhydrides. All of the reactions报道了一种在 Zn(ClO4)2·6H2O 作为催化剂的情况下用酸酐酰化醇的新方案。已证明 Zn( )2·6H2O 的活性优于干燥的 Mg( )2 和金属三氟甲磺酸盐。其效率允许反应性较差的底物(例如位阻叔醇和芳香酸酐)之间发生反应。所有反应均以1:1.05的醇/酸酐比进行。从实用和经济的角度来看,这些条件非常方便,因为它们避免了试剂的浪费并允许简单的后处理程序。Zn( )2·6H2O 的催化作用对酸酐的活化非常特殊,以至于在酯化过程中对酸敏感的官能团和起始材料的立体化学构型保持不变。在所有情况下,酰化产物都是以纯形式定量获得的。(© Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, Germany, 2003)
-
Bartoli, Giuseppe; Bosco, Marcella; Dalpozzo, Renato, Synlett, 2003, # 1, p. 39 - 42作者:Bartoli, Giuseppe、Bosco, Marcella、Dalpozzo, Renato、Marcantoni, Enrico、Massaccesi, Massimo、Rinaldi, Samuele、Sambri, LetiziaDOI:——日期:——
-
The Thermal and Dielectric Properties of Crystalline Long‐Chain Acetates作者:R. J. Meakins、Joan W. MulleyDOI:10.1063/1.1698720日期:1953.11This investigation deals with the normal long-chain acetates with 18, 20, 24, and 28 carbon atoms. When crystallized from the melt, these compounds first give the transparent α-phase which gradually changes to the white, opaque β-phase on standing or on cooling below a certain temperature. It is found that the acetates in the α-phase all give large dielectric absorption, whereas, in the β-phase the absorption is small, or, in some cases, negligible. Of the long-chain compounds so far investigated, the acetates in the α-phase are unique in giving two absorption regions which appear to be the result of the rotational transitions of complete molecules. The maxima for the two regions are separated by about 7 decades in frequency. Measurements of the lower frequency absorption at various temperatures show that the associated energy barrier is very large compared with previous values for compounds of similar chain-length. To explain these results it is suggested that a molecule rotating in the crystal lattice of a long-chain compound possesses four positions of equilibrium instead of two, as previously proposed. In addition to the two absorption regions mentioned above, the long-chain acetates in the α-phase give further absorption at still higher frequencies, which is probably caused by independent orientation of the polar groups near the ends of the molecular chains.
-
STEPHANOU, EURIPIDES G., FRESENIUS J. ANAL. CHEM., 339,(1991) N0, C. 780-784作者:STEPHANOU, EURIPIDES G.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
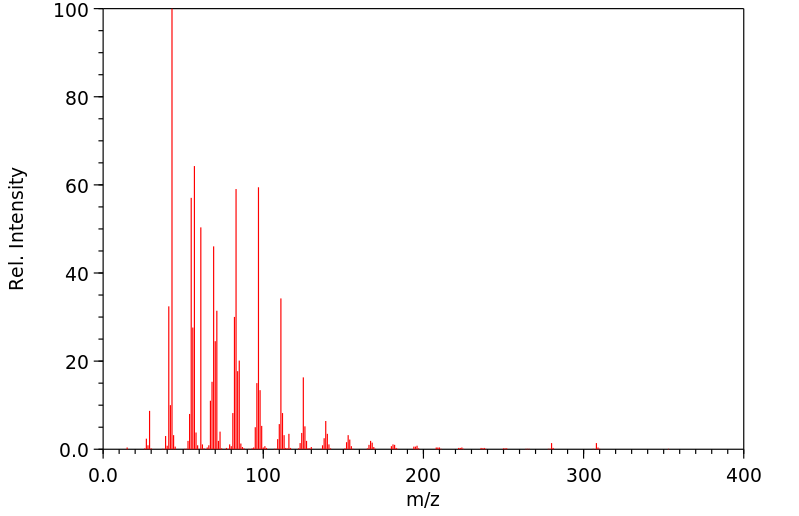
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯