乙酰紫草素 | 24502-78-1
中文名称
乙酰紫草素
中文别名
——
英文名称
acetylshikonin
英文别名
(+)-acetic acid 1-(5,8-dihydroxy-1,4-dioxo-1,4-dihydronaphthalen-2-yl)-4-methylpent-3-enyl ester;(+)-1-(5,8-dihydroxy-1,4-dioxo-1,4-dihydronaphthalen-2-yl)-4-methylpent-3-en-1-yl acetate;2-[1-(acetyloxy)-4-methyl-3-pentenyl]naphthazarin;1'O-acetylshikonin;1'-acetylshikonin;shikonin acetate;(R)-1-(5,8-Dihydroxy-1,4-dioxo-1,4-dihydronaphthalen-2-yl)-4-methylpent-3-en-1-yl acetate;[(1R)-1-(5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl] acetate
CAS
24502-78-1
化学式
C18H18O6
mdl
——
分子量
330.337
InChiKey
WNFXUXZJJKTDOZ-OAHLLOKOSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:86°C
-
沸点:553.2±50.0 °C(Predicted)
-
密度:1.326±0.06 g/cm3(Predicted)
-
溶解度:溶于氯仿、二氯甲烷、乙酸乙酯、DMSO、丙酮等。
-
LogP:2.700 (est)
计算性质
-
辛醇/水分配系数(LogP):3.5
-
重原子数:24
-
可旋转键数:5
-
环数:2.0
-
sp3杂化的碳原子比例:0.28
-
拓扑面积:101
-
氢给体数:2
-
氢受体数:6
安全信息
-
安全说明:S26,S36/37/39
-
危险类别码:R36/37/38
-
储存条件:存于室温、干燥且密封的环境中。
SDS
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 紫草素 shikonin 517-89-5 C16H16O5 288.3 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 丙酰紫草素 [(1R)-1-(5,8-dihydroxy-1,4-dioxo-2-naphthyl)-4-methylpent-3-enyl] propanoate 162283-70-7 C19H20O6 344.364 —— 2-[1-(butyryloxy)-4-methyl-3-pentenyl]naphthazarin —— C20H22O6 358.391 异戊酰紫草素 isovalerylshikonin 52387-14-1 C21H24O6 372.418 —— valerylshikonin —— C21H24O6 372.418 —— [(1R)-1-(5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl] pent-4-enoate —— C21H22O6 370.402 —— (R)-1-(1,4-dihydro-5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl benzoate —— C23H20O6 392.408 —— (+/-)-cycloshikonin 77386-93-7 C16H16O5 288.3 紫草素 shikonin 517-89-5 C16H16O5 288.3
反应信息
-
作为反应物:描述:参考文献:名称:Human ACAT inhibitory effects of shikonin derivatives from Lithospermum erythrorhizon摘要:Three naphthoquinones were isolated by bioassay-guided fractionation from the CHCl3 extracts of roots of Lithospermum erythrorhizon. They were identified as acetylshikonin (1), isobutyrylshikonin (2), and (3-hydroxyisovalerylshikonin (3) on the basis of their spectroscopic analyses. The compounds 1-3 were tested for their inhibitory activities against human ACAT-1 (hACAT-1) or human ACAT-2 (hACAT-2). Compound 2 preferentially inhibited hACAT-2 (IC50 = 57.5 wM) than hACAT-1 (32% at 120 mu M), whereas compounds 1 and 3 showed weak inhibitory activities in both hACAT-1 and -2. To develop more potent hACAT inhibitor, shikonin derivatives (5-11) were synthesized by semi-synthesis of shikonin (4), which was prepared by hydrolysis of 1-3. Among them, compounds 5 and 7 exhibited the strong inhibitory activities against hACAT-1 and -2. Furthermore, we demonstrated that compound 7 behaved as a potent ACAT inhibitor in not only in vitro assay system but also cell-based assay system. (c) 2006 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmcl.2006.11.024
-
作为产物:描述:参考文献:名称:氟酰基紫草素抗癌剂的设计与合成摘要:合成了一系列在紫草素侧链上被各种氟化羧酸选择性酰化的紫草素衍生物,并对其抗癌活性进行了评估,其中首次报道了八种化合物。在所有测试的化合物中,化合物S7对B16-F10(恶性黑色素瘤细胞),MG63(人骨肉瘤细胞)和A549(肺癌细胞)显示出最有效的抗癌活性,IC 50为0.39±0.01、0.72±0.04和0.58 ±0.02 µmol / L。进行化合物S7的对接模拟以将S7定位在微管蛋白活性位点,以确定可能的结合构象。所有结果表明,化合物S7可能是潜在的抗癌药。手性25:757–762,2013。©2013 Wiley Periodicals,Inc.DOI:10.1002/chir.22209
文献信息
-
Formation of stereoisomeric mixtures of naphthoquinone derivatives in Echium lycopsis callus cultures作者:Hiroshi Fukui、Minoru Tsukada、Hajime Mizukami、Mamoru TabataDOI:10.1016/0031-9422(83)83022-2日期:1983.1esterified derivative, although the overall ratio for the total derivatives was ca 1:1. On the other hand, all the corresponding derivatives produced by Lithospermum cultures were primarily of the R -form. It was also demonstrated that pigment formation in Echium cultures was inhibited by either white or blue light as well as by the synthetic auxin 2,4-D as in the case of Lithospermum cultures.
-
[EN] METHODS FOR TREATING CANCER<br/>[FR] MÉTHODES DE TRAITEMENT DU CANCER申请人:SCORPION THERAPEUTICS INC公开号:WO2022094271A1公开(公告)日:2022-05-05This disclosure provides chemical entities of formula (I) (e.g., a compound or a pharmaceutically acceptable salt, and/or hydrate, and/ or cocrystal, and/or drug combination of the compound) that inhibit epidermal growth factor receptor (EGFR, ERBB 1) and/or Human epidermal growth factor receptor 2 (HER2, ERBB2). These chemical entities are useful, e.g., for treating a condition, disease or disorder in which increased (e.g., excessive) EGFR and/or HER2 activation contributes to the pathology and/or symptoms and/or progression of the condition, disease or disorder (e.g., cancer) in a subject (e.g., a human). This disclosure also provides compositions containing the same as well as methods of using and making the same.
-
Method of producing ginsenoside 20 (R)-Rh2 and composition of matter thereof申请人:Young Jeffrey公开号:US20050287230A1公开(公告)日:2005-12-29The present invention is a method of producing ginsenoside comprising the steps of: (a) producing a predetermined quantity of ginseng dialcohol from an extraction process of ginseng dialcohol; (b) reacting the ginseng dialcohol with a predetermined quantity of acetobromo-α-D-glucose under a predetermined condition; and (c) obtaining a predetermined quantity of ginsenoside Rh 2 . The present invention also provides a composition for enhancing the immune system of a living object comprising a quantity of ginsenoside, a quantity of ursolic acid and a quantity of shikonin. The composition may also comprise a quantity of panaxans, astragalan, and/or lentinan. The ginsenoside 20(R)-Rh 2 , ursolic acid, shikonin, panaxans, astragalan and lentinan have an effective range between 0.001 mg/kg and 0.1 mg/kg, 0.5 mg/kg and 10 mg/kg, 0.5 mg/kg and 10 mg/kg, 0.1 mg/kg and 1 mg/kg, 0.1 mg/kg and 1 mg/kg, and 0.1 mg/kg and 1 mg/kg respectively.本发明涉及一种生产人参皂苷的方法,包括以下步骤:(a)从人参二醇的提取过程中生产出预定量的人参二醇;(b)在预定条件下将人参二醇与预定量的乙酰溴-α-D-葡萄糖反应;(c)获得预定量的人参皂苷Rh2。本发明还提供了一种增强生物体免疫系统的组合物,包括一定量的人参皂苷、一定量的熊果酸和一定量的赤芍。该组合物还可以包括一定量的人参皂苷、黄芪苷和/或香菇多糖。人参皂苷20(R)-Rh2、熊果酸、赤芍、人参皂苷、黄芪苷和香菇多糖在0.001 mg/kg至0.1 mg/kg、0.5 mg/kg至10 mg/kg、0.5 mg/kg至10 mg/kg、0.1 mg/kg至1 mg/kg、0.1 mg/kg至1 mg/kg和0.1 mg/kg至1 mg/kg之间具有有效范围。
-
Method of treatment of virus infections using shikonin compounds
-
[EN] USE OF MACROCYCLIC COMPOUNDS IN METHODS OF TREATING CANCER<br/>[FR] UTILISATION DE COMPOSÉS MACROCYCLIQUES DANS DES MÉTHODES DE TRAITEMENT DE CANCER申请人:SCORPION THERAPEUTICS INC公开号:WO2022098992A1公开(公告)日:2022-05-12This disclosure provides chemical entities (e.g., a compound or a pharmaceutically acceptable salt, and/or hydrate, and/or cocrystal, and/or drug combination of the compound) that inhibit epidermal growth factor receptor (EGFR, ERBB1) and/or Human epidermal growth factor receptor 2 (HER2, ERBB2). These chemical entities are useful, e.g., for treating a condition, disease or disorder in which increased (e.g., excessive) EGFR and/or HER2 activation contributes to the pathology and/or symptoms and/or progression of the condition, disease or disorder (e.g., cancer) in a subject (e.g., a human). This disclosure also provides compositions containing the same as well as methods of using and making the same.
表征谱图
-
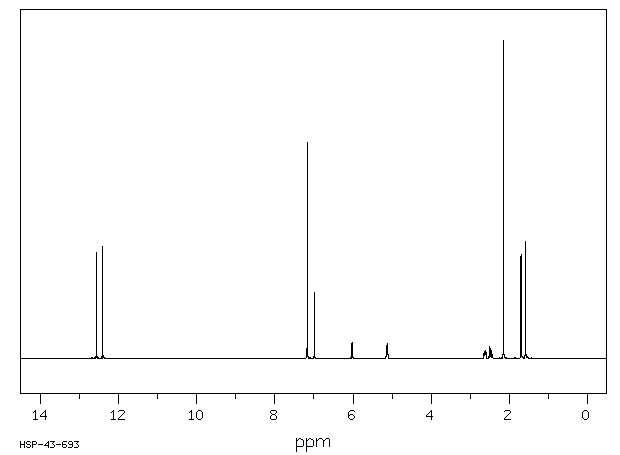
氢谱1HNMR
-
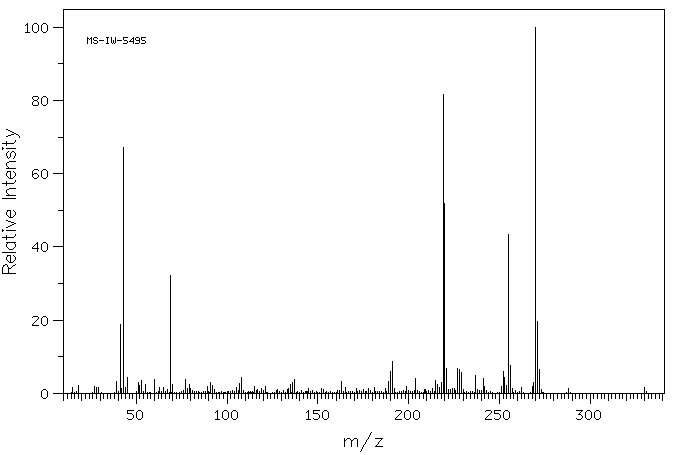
质谱MS
-
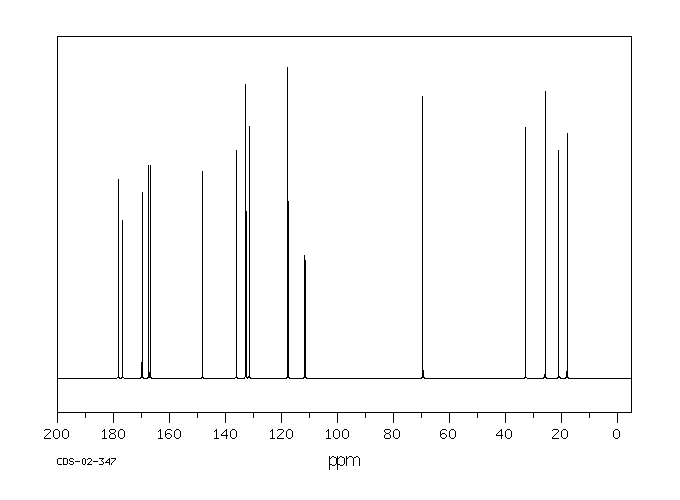
碳谱13CNMR
-
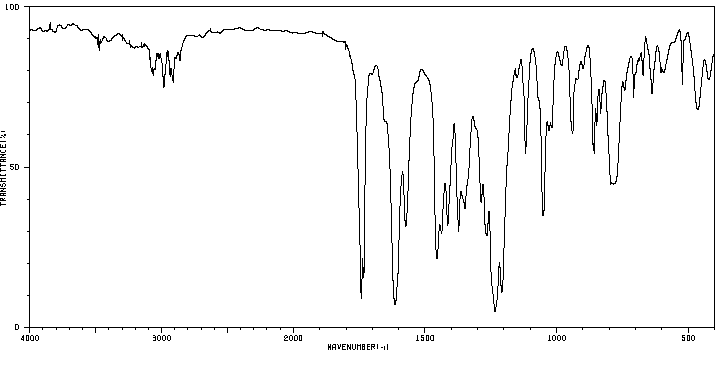
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮