(E)-4-phenylbutan-2-one oxime | 66417-92-3
中文名称
——
中文别名
——
英文名称
(E)-4-phenylbutan-2-one oxime
英文别名
N-[(2Z)-4-Phenylbutan-2-ylidene]hydroxylamine;(NE)-N-(4-phenylbutan-2-ylidene)hydroxylamine
CAS
66417-92-3
化学式
C10H13NO
mdl
——
分子量
163.219
InChiKey
FTYXDGNSLZMOQY-PKNBQFBNSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:12
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:32.6
-
氢给体数:1
-
氢受体数:2
SDS
上下游信息
反应信息
-
作为反应物:描述:(E)-4-phenylbutan-2-one oxime 在 四丁基高铼酸铵 三氟甲磺酸 、 5A molecular sieve 、 四氯苯醌 作用下, 以 1,2-二氯乙烷 为溶剂, 反应 1.0h, 以55%的产率得到2-甲基喹啉参考文献:名称:四丁基高铼酸铵和三氟甲磺酸催化苯乙酮衍生物肟转化为喹啉和氮杂螺三烯酮摘要:在回流的 1,2-二氯乙烷中,用四丁基高铼酸铵、三氟甲磺酸和氯苯醌处理将苯乙酮肟转化为喹啉。氮杂螺三烯酮可由对羟基苯乙基或3-(对羟基苯基)丙基酮肟采用上述方法合成。如此制备的氮杂螺三烯酮通过酸处理转化为喹啉。DOI:10.1246/bcsj.70.965
-
作为产物:描述:参考文献:名称:铱催化肟转移加氢合成N-烷氧基胺和羟胺摘要:发现阳离子铱(Ir)配合物催化肟的转移加氢以得到N-烷氧基胺和羟胺,并且三氟乙酸加速了反应。该协议的实际应用通过克级转化和杀菌剂 furmecyclox (BAS 389F) 的两步合成得到证明,总产量分别为 92% 和 85%。证明了使用手性 Ir 配合物提供手性N-烷氧基胺的不对称方案,但获得的低产率/ee 表明需要进一步开发。DOI:10.1039/d2ob01084d
文献信息
-
Stereospecific synthesis of 1,5-disubstituted tetrazoles from ketoximes via a Beckmann rearrangement facilitated by diphenyl phosphorazidate作者:Kotaro Ishihara、Takayuki Shioiri、Masato MatsugiDOI:10.1016/j.tetlet.2019.04.014日期:2019.5A novel method for the stereospecific synthesis of 1,5-disubstituted tetrazoles from ketoximes via the Beckmann rearrangement was developed using diphenyl phosphorazidate (DPPA) as both the oxime activator and azide source. Various ketoximes were transformed into the corresponding 1,5-disubstituted tetrazoles with exclusive trans-group migration and no E-Z isomerization of the ketoxime. This method
-
A mild system for synthesis of aldoximes and ketoximes in the presence of N-hydroxyphthalimide in aqueous system作者:Xiaoying Jiang、Xiaohe Xu、Yuyan Lin、Yiyan Yan、Pingping Li、Renren Bai、Yuanyuan XieDOI:10.1016/j.tet.2018.08.013日期:2018.10method for synthesis of oximes from aldehydes or ketones with N-hydroxyphthalimide or N-hydroxysuccinimide in water has been described. It is the first time to utilize NHPI as an oximation reagent to synthesize aldoximes and ketoximes from the corresponding organic carbonyl compounds without other reagents. The reaction tolerates various functional groups and affords the corresponding oximes in 76%–98% yields
-
Synthesis of Quinolines and 2<i>H</i>-Dihydropyrroles by Nucleophilic Substitution at the Nitrogen Atom of Oxime Derivatives作者:Koichi Narasaka、Mitsuru Kitamura、Masayuki Yoshida、Takashi KikuchiDOI:10.1055/s-2003-42439日期:——Isomerization of oxime derivatives was researched in detail to find out the methods for the syn-anti isomerization of O-substituted oximes. Based on these findings were developed simple methods for the preparation of aza-heterocycles from both stereoisomers of oximes. Quinolines were synthesized from β-aryl ketone oximes by treatment with trifluoroacetic anhydride and 4-chloranil. γ,δ-Unsaturated O-methoxyacetyloximes were transformed to 2H-dihydropyrroles by reaction with methoxy-acetic acid.
-
Beckmann Rearrangement of Oximes Catalyzed with Tetrabutylammonium Perrhenate and Trifluoromethanesulfonic Acid作者:Hiroyuki Kusama、Yuko Yamashita、Koichi NarasakaDOI:10.1246/bcsj.68.373日期:1995.1The Beckmann rearrangement of oximes is catalyzed by a combined use of tetrabutylammonium perrhenate (Bu4NReO4) and trifluoromethanesulfonic acid in nitromethane under azeotropic conditions, giving amides in high yield. By employing this catalytic system, amides can be prepared directly from ketones and hydroxylamine hydrochloride.
-
Three component hydroxyletherification and hydroxylazidation of (trifluoromethyl)alkenes: access to α-trifluoromethyl β-heteroatom substituted tertiary alcohols作者:Hao Zeng、Chuanle Zhu、Chi Liu、Yingying Cai、Fulin Chen、Huanfeng JiangDOI:10.1039/d0cc02550j日期:——
The three component hydroxyletherification and hydroxylazidation reactions of (trifluoromethyl)alkenes are reported, providing various useful α-trifluoromethyl β-heteroatom substituted tertiary alcohols in high yields.
表征谱图
-
氢谱1HNMR
-
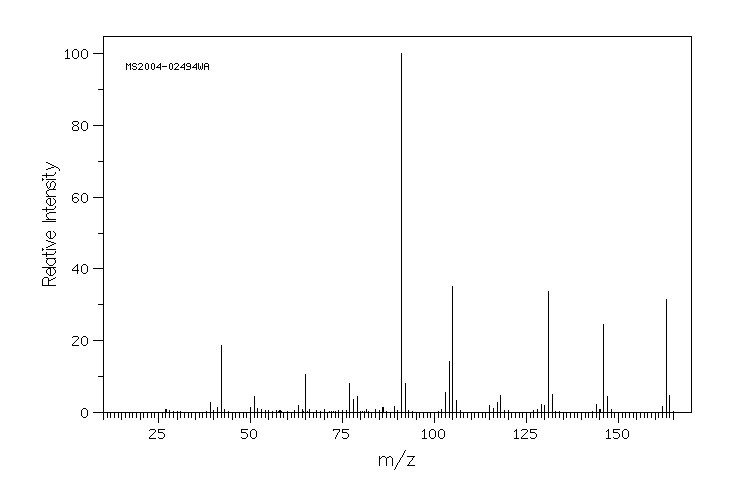
质谱MS
-
碳谱13CNMR
-
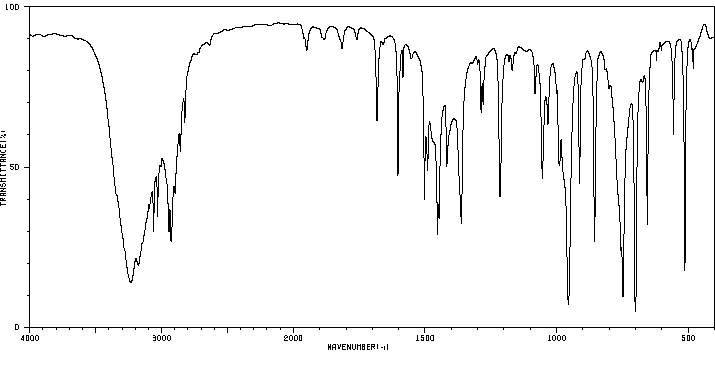
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫