Succinanilid | 15510-09-5
中文名称
——
中文别名
——
英文名称
Succinanilid
英文别名
Bernsteinsaeure-dianilid;N,N'-diphenylsuccinamide;Succinanilide;N,N'-diphenylbutanediamide
CAS
15510-09-5
化学式
C16H16N2O2
mdl
——
分子量
268.315
InChiKey
NOEOAZMRAXYIEK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:146-147 °C(Solv: methanol (67-56-1))
-
沸点:570.4±33.0 °C(Predicted)
-
密度:1.239±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:20
-
可旋转键数:5
-
环数:2.0
-
sp3杂化的碳原子比例:0.12
-
拓扑面积:58.2
-
氢给体数:2
-
氢受体数:2
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 琥珀酸一酰苯胺 N-phenyl-succinamic acid 102-14-7 C10H11NO3 193.202 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— N-phenylsuccinamide 15386-95-5 C10H12N2O2 192.217 琥珀酸一酰苯胺 N-phenyl-succinamic acid 102-14-7 C10H11NO3 193.202 —— N,N'-bis(4-nitrophenyl)butanediamide 42348-39-0 C16H14N4O6 358.31 N-苯丁二醯亞胺 N-phenylmaleimide 83-25-0 C10H9NO2 175.187
反应信息
-
作为反应物:描述:参考文献:名称:Menschutkin, Justus Liebigs Annalen der Chemie, 1872, vol. 162, p. 166摘要:DOI:
-
作为产物:参考文献:名称:Staudinger; Kreis, Helvetica Chimica Acta, 1923, vol. 6, p. 324摘要:DOI:
文献信息
-
Simultaneous Quantification of Metabolites Involved in Central Carbon and Energy Metabolism Using Reversed-Phase Liquid Chromatography−Mass Spectrometry and in Vitro <sup>13</sup>C Labeling作者:Wen-Chu Yang、Miroslav Sedlak、Fred E. Regnier、Nathan Mosier、Nancy Ho、Jiri AdamecDOI:10.1021/ac801693c日期:2008.12.15Comprehensive analysis of intracellular metabolites is a critical component of elucidating cellular processes. Although the resolution and flexibility of reversed-phase liquid chromatography−mass spectrometry (RPLC−MS) makes it one of the most powerful analytical tools for metabolite analysis, the structural diversity of even the simplest metabolome provides a formidable analytical challenge. Here we describe a robust RPLC−MS method for identification and quantification of a diverse group of metabolites ranging from sugars, phosphosugars, and carboxylic acids to phosphocarboxylics acids, nucleotides, and coenzymes. This method is based on in vitro derivatization with a 13C-labeled tag that allows internal standard based quantification and enables separation of structural isomer pairs like glucose 6-phosphate and fructose 6-phosphate in a single chromatographic run. Calibration curves for individual metabolites showed linearity ranging over more than 2 orders of magnitude with correlation coefficients of R2 > 0.9975. The detection limits at a signal-to-noise ratio of 3 were below 1.0 μM (20 pmol) for most compounds. Thirty common metabolites involved in glycolysis, the pentose phosphate pathway, and tricarboxylic acid cycle were identified and quantified from yeast lysate with a relative standard deviation of less than 10%.详细分析细胞内代谢物是阐明细胞过程的关键组成部分。尽管反相液相色谱-质谱联用(RPLC-MS)的分辨率和灵活性使其成为代谢物分析中最强大的分析工具之一,但即使是简单的代谢组,其结构的多样性也带来了巨大的分析挑战。本文描述了一种稳健的RPLC-MS方法,用于鉴定和定量一系列广泛的代谢物,包括糖类、磷酸糖、羧酸、磷酸羧酸、核苷酸和辅酶。该方法基于使用13C标记标签的体外衍生化,允许基于内标的定量,并能在单次色谱运行中分离结构异构体对,如葡萄糖6-磷酸和果糖6-磷酸。单个代谢物的校准曲线显示线性范围超过两个数量级,相关系数R² > 0.9975。大多数化合物的信噪比为3的检测限低于1.0 μM(20 pmol)。从酵母裂解液中鉴定和定量了涉及糖酵解、戊糖磷酸途径和三羧酸循环的30种常见代谢物,相对标准偏差小于10%。
-
One-pot mechanosynthesis of aromatic amides and dipeptides from carboxylic acids and amines作者:Vjekoslav Štrukil、Boris Bartolec、Tomislav Portada、Ivica Đilović、Ivan Halasz、Davor MargetićDOI:10.1039/c2cc36613d日期:——Environmentally friendly one-pot synthesis of amides, bis-amides and dipeptides by mechanochemical carbodiimide-mediated coupling of carboxylic acids and amines is described; high reaction yields and simple aqueous work-up allow for the clean, practical and fast preparation of a variety of compounds containing the amide bond from readily accessible reagents.
-
Nucleophilic Substitution at the Guanidine Carbon Center via Guanidine Cyclic Diimide Activation作者:Taeyang An、Yan LeeDOI:10.1021/acs.orglett.1c03473日期:2021.12.3of the guanidine carbon centers, nucleophilic reactions at these sites have been underdeveloped because of the resonance stabilization of the guanidine group. We propose a guanidine C–N bond substitution strategy entailing the formation of guanidine cyclic diimide (GCDI) structures, which effectively destabilize the resonance structure of the guanidine group. In the presence of acid additives, the guanidine
-
Synthesis of N,N’-Diarylalkanediamides and Their Antimycobacterial and Antialgal Activity作者:Lenka Kubicova、Karel Waisser、Jiri Kunes、Katarina Kralova、Zelmira Odlerova、Milan Slosarek、Jiri Janota、Zbynek SvobodaDOI:10.3390/50500714日期:——A set of N,N'-diarylalkanediamides was synthesized. The compounds were tested for their antimycobacterial and antialgal activity. The antimycobacterial activity of N,N'-diarylalkanediamides depends on the lipophilicity of the respective acid. Antimycobacterially active substances were found only in the series of N,N'-diarylethanediamides and N,N'-diarylbutanediamides. Other compounds (derivatives of pentane-, hexane-, octane- and nonanediamide) were inactive against various strains of mycobacteria. The compounds inhibited growth and chlorophyll production in Chlorella vulgaris. Their relatively low antialgal activity is probably connected with their lowered aqueous solubility, and hence by a restricted passage of the inhibitor through the hydrophilic regions of thylakoid membranes.
-
N,N'-substituted-1,3-diamino-2-hydroxypropane derivatives申请人:John Varghese公开号:US20070213316A1公开(公告)日:2007-09-13Disclosed are compounds of the formula wherein the variables R N , R C , R 1 , R 25 , R 2 , and R 3 are as defined herein. These compounds have activity as inhibitors of beta-secretase and are therefore useful in treating a variety of discorders such as Alzheimer's Disease.本发明揭示了以下式子的化合物:其中变量RN,RC,R1,R25,R2和R3如本文所定义。这些化合物具有抑制β-分泌酶的活性,因此可用于治疗多种疾病,例如阿尔茨海默病。
表征谱图
-
氢谱1HNMR
-
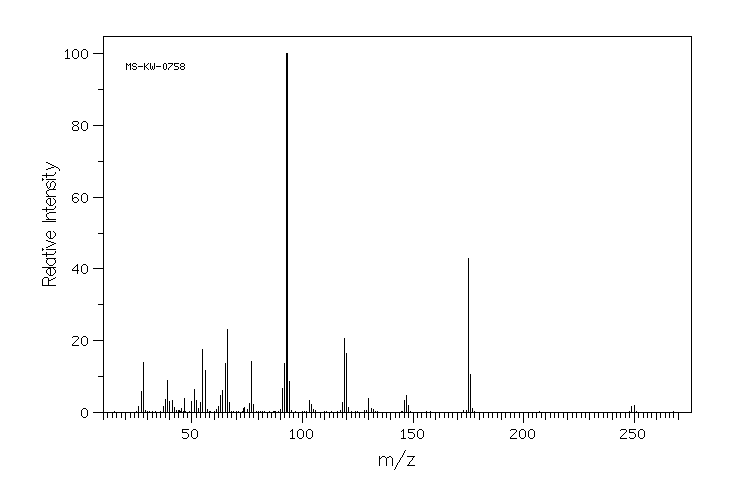
质谱MS
-
碳谱13CNMR
-
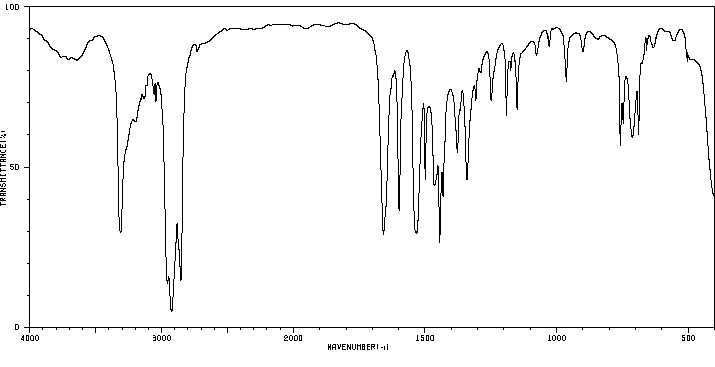
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫