八氢-并环戊二烯 | 694-72-4
中文名称
八氢-并环戊二烯
中文别名
——
英文名称
bicyclo[3.3.0]octane
英文别名
bicyclo<3.3.0>octane;Bicylo<3.3.0>octan;Pentalene, octahydro-;1,2,3,3a,4,5,6,6a-octahydropentalene
CAS
694-72-4
化学式
C8H14
mdl
MFCD00060882
分子量
110.199
InChiKey
AEBWATHAIVJLTA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-30 °C
-
沸点:132 °C(Press: 762 Torr)
-
密度:0.896±0.06 g/cm3(Predicted)
-
大气OH速率常数:1.10e-11 cm3/molecule*sec
-
保留指数:859.9;865.2;870;858;863;865;869;874;858;870;870;891
计算性质
-
辛醇/水分配系数(LogP):3.5
-
重原子数:8
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2912299000
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:Synthesis, spectral and structural characterization of Ni(II), Cu(II), Zn(II), Cd(II) and Hg(II) complexes with 2-mercapto-5-methyl-1,3,4-thiadiazole: A Zn(II) complex acting as a new sensitive and selective fluorescent probe for the detection of Hg2+ in H2O–MeOH medium摘要:Five new complexes, [Ni(mthd)(2)(py)(2)] (1), [Cu(en)(2)](mthd)(2) (2), [H(2)en][Hg(mthd)(3)](2)center dot 2H(2)O (3), [Cd(mthd)(2)(o-phen)(2)](2)center dot H2O (4) and [Zn(mthd)(2)(bpy)] (5) (Hmthd = 2-mercapto-5-methyl-1,3,4-thiadiazole), have been synthesized. All the complexes have been fully characterized by various techniques: elemental analyses, IR, electronic and fluorescent spectral data. The ligand is present in the deprotonated thiol form in the complexes [Cu(en)(2)](mthd)(2) (2) and [Cd(mthd)(2)(o-phen)(2)]2 center dot H2O (4). In complex 2, the ligand is ionically bonded, whereas it is covalently bonded through the sulfur in complex 4. In [Ni(mthd)(2)(py)(2)] (1) the ligand is N,S chelating bidentate bonded through the thiol sulfur and the thiadiazole ring nitrogen adjacent to it, forming a four membered chelate ring. The ligand is covalently bonded through the deprotonated thiadiazole ring nitrogen adjacent to the thiol sulfur in [Zn(mthd)(2)(bpy)] (5). The complex anion in [H(2)en][Hg(mthd)(3)](2)center dot 2H(2)O (3) has a triangular planar geometry, with bonding through the deprotonated thiolato sulfur atoms from the three ligands. [Zn(mthd)(2)(bpy)] (5) is highly fluorescent as compared to the other complexes and has been further used as a metal probe for sensing of Hg2+ in H2O-MeOH solution. Complex 5, upon interaction with Hg2+, shows a hypochromic shift in the absorption spectra whereas the emission spectra exhibited 75% quenching fluorescence behavior. The electrochemical studies also suggest the interaction of Hg(II) with the Zn(II) complex, probably via the free thione sulfur. (C) 2013 Elsevier Ltd. All rights reserved.DOI:10.1016/j.poly.2013.07.027
-
作为产物:参考文献:名称:Preparatively useful dehydrogenative method for dodecahedrane synthesis摘要:DOI:10.1021/ja00267a067
文献信息
-
High temperature bromination Part XXIII: Bromination of octahydro-1H-indene and octahydro-1H-4,7-methanoindene作者:Melek Sermin Ozer、Benan Kilbas、Metin BalciDOI:10.3998/ark.5550190.p008.195日期:——photobromination of octahydro-1H-indene and octahydro-1H-4,7-methanoindene were investigated. Three isomeric tetrabromides (1,3,4,7-tetrabromo-2,3,4,5,6,7-hexahydro-1Hindene) were formed along with a smaller amount of tribromoindane and a pentabromide by thermal bromination of octahydro-1H-indene. The thermodynamically most stable isomers were formed. Morover, thermal and photochemical bromination of octahydro-1H-4
-
Highly Active Superbulky Alkaline Earth Metal Amide Catalysts for Hydrogenation of Challenging Alkenes and Aromatic Rings作者:Johannes Martin、Christian Knüpfer、Jonathan Eyselein、Christian Färber、Samuel Grams、Jens Langer、Katharina Thum、Michael Wiesinger、Sjoerd HarderDOI:10.1002/anie.202001160日期:2020.6.2series of bulky alkaline earth (Ae) metal amide complexes have been prepared: Ae[N(TRIP)2 ]2 (1-Ae) and Ae[N(TRIP)(DIPP)]2 (2-Ae) (Ae=Mg, Ca, Sr, Ba; TRIP=SiiPr3 , DIPP=2,6-diisopropylphenyl). While monomeric 1-Ca was already known, the new complexes have been structurally characterized. Monomers 1-Ae are highly linear while the monomers 2-Ae are slightly bent. The bulkier amide complexes 1-Ae are by far制备了两个系列的大体积碱土金属 (Ae) 金属酰胺配合物:Ae[N(TRIP)2 ]2 (1-Ae) 和 Ae[N(TRIP)(DIPP)]2 (2-Ae) (Ae= Mg、Ca、Sr、Ba;TRIP=SiiPr3,DIPP=2,6-二异丙基苯基)。虽然单体 1-Ca 已为人所知,但新复合物的结构已得到表征。单体1-Ae是高度线性的,而单体2-Ae是轻微弯曲的。体积较大的酰胺配合物 1-Ae 是迄今为止烯烃加氢中最活跃的催化剂,其活性从 Mg 增加到 Ba。催化剂 1-Ba 可以还原环己烯或 3-己烯等内烯烃以及 1-Me-环己烯或四苯乙烯等极具挑战性的底物。它还对芳烃氢化还原蒽和萘(即使被烷基取代)以及联苯具有活性。苯可以还原为环己烷,但未达到完全转化。催化氢化的第一步是形成(酰胺)AeH 物质,它可以形成更大的聚集体。增加酰胺配体的体积会降低聚集体尺寸,但尚不清楚真正的催化剂是什么。 DFT
-
Catalytic dehydrogenation of cyclooctane with titanium, zirconium and hafnium metallocene complexes作者:Sandra Taubmann、Christine E. Denner、Helmut G. AltDOI:10.1016/j.jorganchem.2009.01.048日期:2009.6structure. Generally, complexes with low steric demands and MAO as cocatalyst gave the highest activities. The comparison of different π-ligands resulted in the following activity order: cyclopentadienyl > indenyl > fluorenyl. The influence of σ-ligands and n-donor ligands gave the following activity order: –Cl > –PMe3 > –CH2Ph > –(CH2)4CH3 > –NPh3. The activities depended on the nature of the cocatalyst茂金属配合物与助催化剂(如甲基铝氧烷(MAO))的结合不仅是烯烃聚合的极好催化剂,而且还是在均相(高压釜)和非均相(固定床反应器)反应中活化烷烃的合适催化剂。催化剂的活性取决于温度,助催化剂,添加剂,中心金属和配体结构。通常,低空间需求的配合物和MAO作为助催化剂的活性最高。不同π-配体的比较导致以下活性顺序:环戊二烯基>茚基>芴基。σ-配体和n-给体配体的影响给出以下活性顺序:–Cl> –PMe 3 > –CH 2 Ph> –(CH 2)4 CH 3 > –NPh 3。活性取决于助催化剂的性质并以下列顺序降低:MAOAOAlMe 3 > AlEt 3。铝粉和路易斯碱NPh 3的添加提高了Cp 2 ZrCl 2 / MAO催化剂的活性。Cp 2 ZrCl 2 / MAO / NPh 3催化剂在300°C的16 h内,均相反应具有458次转换的最高活性。Cp 2 ZrCl 2 / MAO
-
Catalytic thermal and photo-induced CH and CC activation reactions of alkanes with ansa amido functionalized half-sandwich complexes and methylalumoxane作者:H.G. Alt、Christine E. DennerDOI:10.1016/j.jorganchem.2009.06.018日期:2009.9Zr, Hf) for CH and CC activation reactions is completely unknown in the literature. In contrast to the dehydrogenation reactions of cyclooctane and the metallocene complexes of the group 4 metals, where the zirconocene complexes give higher TONs than the titanocene complexes the ansa amido functionalized titanium complexes give more than two times higher TONs than the corresponding Zr or Hf complexes在文献中,烷烃的大多数脱氢反应被描述为环辛烷的CH活化反应。已发现CHA活化反应的最佳结果是MAO活化的茂金属络合物与环辛烷在300°C以上的温度下反应。 Ind'Si(Me)2NtBuMCl2(Ind'=单取代的茚基)类型的ansa酰胺基官能化半三明治化合物的应用;CH和CC活化反应的M = Ti,Zr,Hf)在文献中是完全未知的。 与环辛烷和第4组金属的茂金属配合物的脱氢反应相反,其中茂茂锆配合物给出的TONs高于二茂钛配合物,而ansa酰胺基官能化钛配合物给出的TONs则高出相应的Zr或Hf配合物两倍以上。ansa酰胺基官能化的配体会增加Ti络合物的TON,降低Zr络合物的TON。 与茂金属配合物相反,钛的ansa酰胺基官能化的二氯化物配合物也比相应的Zr配合物具有更高的活性。已知有机金属钛,锆和ha(IV)化合物的光解可产生M(III)自由基。与相应的Zr和Hf金属化合物相比,活性Ti金属中心的形成更容易。
-
Dehydrogenation of Cycloalkanes by Suspended Platinum Catalysts作者:Shunichi Hama、Xiaomei Li、Kiyoshi Yukawa、Yasukazu SaitoDOI:10.1246/cl.1992.2463日期:1992.12Heterogenized cluster complex [Pt3(CO)6]52− and reduced platinum metal have catalyzed cyclooctane dehydrogenation at comparable rates, whereas dehydrogeno-aromatization of ethylcyclohexane proceeded predominantly with the latter. Active-site ensembles for the formation of cyclooctene, bicyclooctane and aromatics were discussed on reference to the appropriate stretch of platinum metal atoms.
表征谱图
-
氢谱1HNMR
-
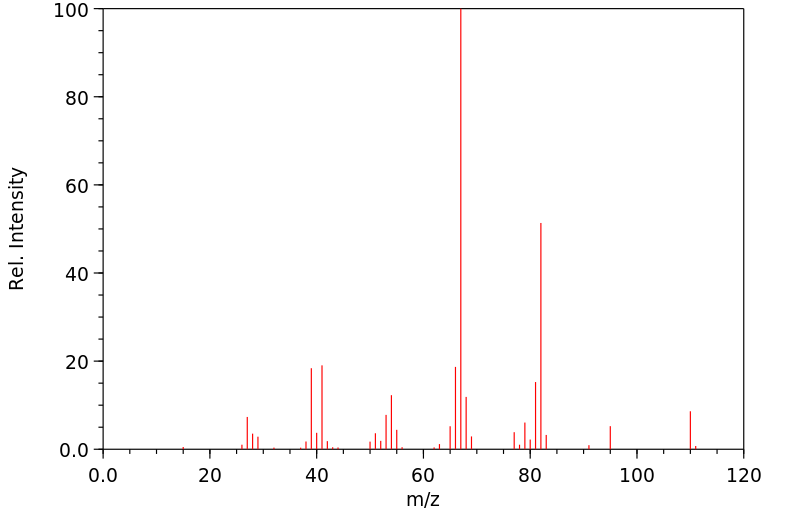
质谱MS
-
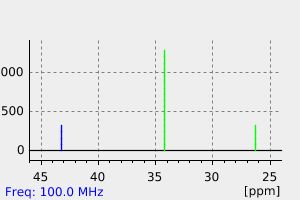
碳谱13CNMR
-
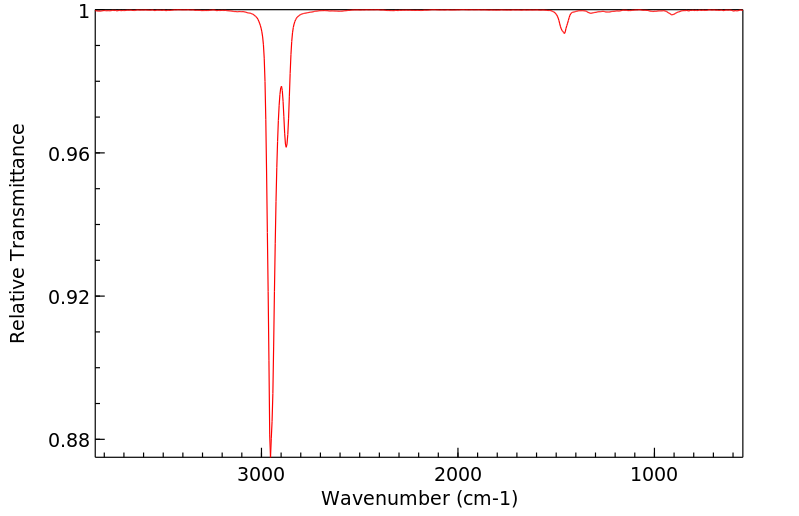
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
降冰片烯
金刚烷-D16
金刚烷
螺戊烷
螺二環己烷
螺[5.6]十二烷
螺[5.5]十一碳-4-烯
螺[5.2]辛-2-烯
螺[4.5]癸烷
螺[4.4]壬-8-烯
螺[3.4]辛烷
螺[3.4]辛-7-烯
螺[3.3]庚-2,5-二烯
螺[2.5]辛烷
螺[2.5]辛-7-烯
螺[2.5]辛-5,7-二烯
螺[2.4]庚-4,6-二烯
螺[2.4]庚-1-烯
螺[2.3]己-1-烯
螺[2.2]戊-1-烯
螺<二环<2.2.2>辛-5-烯-2,1'-环丙烷>
螺<4.4>壬-1,3,7-三烯
螺<4.4>壬-1,3,6,8-四烯
螺(4.4)壬烷
螺(4.4)壬-1,3-二烯
螺(3.4)辛-5,7-二烯
trans-perhydroazulene
萘烷
萘,1,2,3,4,4a,8a-六氢-,顺-
美罗培南中间体F9
篮烷
立方烷
氨基甲硫酸,二甲基-,O,O-(3,3-二甲基1,1-联苯基-2,2-二基)酯
棱晶烷
杜瓦苯
新戊基-1金刚烷
抗氧化剂TH-CPL
庚搭烯
四螺[2.0.2:0.2:0.2:0]十二烷
四螺[2.0.0.0.2.1.1.1]十一烷,顺-
四环己基铅
四环[5.3.0.0<2,6>.0<3,10>]癸-4,8-二烯
四环[5.3.0.0(2,6).0(3,10)]癸烷
四环[4.4.0.02,10.03,7]癸-4,8-二烯
四环[4.2.2.26,5.01,6]十二烷
四环[4.2.0.02,5.03,8]辛烷
四环[3.3.0.02,4.03,6]辛-7-烯
四环<5.3.1.02,6.04,9>十一烷
四环(8.2.2.22,5.26,9)十八碳-1,5,9-三烯
四环(4.1.0.0(2,4).0(3,5))庚烷