4-氯苄基硫代异氰酸酯 | 3694-45-9
中文名称
4-氯苄基硫代异氰酸酯
中文别名
4-氯苄基异硫氰酸酯;4-氯苄基硫氰酸酯
英文名称
4-chlorobenzylisothiocyanate
英文别名
1-chloro-4-(isothiocyanatomethyl)benzene;4-Chlorobenzyl isothiocyanate
CAS
3694-45-9
化学式
C8H6ClNS
mdl
——
分子量
183.661
InChiKey
DEHXIHUIYSXZNH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:170 °C
-
密度:1,27 g/cm3
-
闪点:169-170°C/25mm
-
稳定性/保质期:
在常温常压下保持稳定。
计算性质
-
辛醇/水分配系数(LogP):3.8
-
重原子数:11
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.12
-
拓扑面积:44.4
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险等级:6.1
-
危险品标志:T
-
危险类别码:R20/21/22,R36/37/38
-
危险品运输编号:2810
-
RTECS号:NX8470000
-
海关编码:2930909090
-
包装等级:II
-
危险类别:6.1
-
安全说明:S26,S36/37/39
-
储存条件:常温下,应避光、存放在通风干燥处,并密封保存。
SDS
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 对氯苄胺 4-chlorobenzylamine 104-86-9 C7H8ClN 141.6 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 对氯苄胺 4-chlorobenzylamine 104-86-9 C7H8ClN 141.6 1-(4-氯苄基)-2-硫脲 1-(4-chlorobenzyl)thiourea 24827-37-0 C8H9ClN2S 200.692
反应信息
-
作为反应物:描述:参考文献:名称:Bacteriostats. II.1 The Chemical and Bacteriostatic Properties of Isothiocyanates and their Derivatives摘要:DOI:10.1021/ja01525a057
-
作为产物:描述:参考文献:名称:New potent imidazoisoquinolinone derivatives as anti-Trypanosoma cruzi agents: Biological evaluation and structure–activity relationships摘要:A series of novel benzoimidazo andN-aryl-5-oxo-imidazo[1,2-b] isoquinoline-10-carbothioamides was developed. All the compounds were evaluated for their in vitro action against the epimastigote form of Trypanosoma cruzi. Four of them showed higher activity than Nifurtimox. Their unspecific cytotoxicity was evaluated using HeLa and L6 cells, being non-toxic at concentrations at least 15 and 200 times higher than that of T. cruzi IC50. To gain insight into the mechanism of action, their DNA binding properties and reactivity with glutathione were studied, and QSAR study was performed. (c) 2009 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmc.2009.01.011
文献信息
-
Na<sub>2</sub>S<sub>2</sub>O<sub>8</sub>-mediated efficient synthesis of isothiocyanates from primary amines in water作者:Zhicheng Fu、Wenhao Yuan、Ning Chen、Zhanhui Yang、Jiaxi XuDOI:10.1039/c8gc02261e日期:——We have developed two green, practical, and efficient procedures, including a one-pot one, to synthesize isothiocyanates from amines and carbon disulfide via desulfurization with sodium persulfate. Water is used as the solvent. Basic conditions are necessary for good chemoselectivity for isothiocyanates. Structurally diverse linear and branched alkyl amines and aryl amines are readily converted to isothiocyanates
-
Design, Synthesis, and Structure−Activity Relationships of Novel Non-Imidazole Histamine H<sub>3</sub> Receptor Antagonists作者:Ian D. Linney、Ildiko M. Buck、Elaine A. Harper、S. Barret Kalindjian、Michael J. Pether、Nigel P. Shankley、Gillian F. Watt、Paul T. WrightDOI:10.1021/jm990952j日期:2000.6.18.38 +/- 0.10), 31, exhibit high affinity for the histamine H(3) receptor. Antagonists 30 and 31 demonstrate significant selectivity over the other histamine, H(1) and H(2), receptor subtypes and a 100-fold selectivity in the sigma(1) binding assay. Compounds 30and 31 are the most potent, selective non-imidazole histamine H(3) receptor antagonists reported in the literature to date.基于低亲和力配体dimaprit(pK(I)7.32 +/- 0.12,pK(B)5.93 +/- 0.17)已经制备了新型,有效和选择性的非咪唑组胺H(3)受体拮抗剂。详细的结构活性研究表明,N-(4-氯苄基)-N-(6-吡咯烷-1-基己基)胍(pK(I)8.38 +/- 0.21,pK(B)8.39 +/- 0.13), 30和N-(4-氯苄基)-N-(7-吡咯烷-1-基庚基)胍(pK(I)8.78 +/- 0.12,pK(B)8.38 +/- 0.10)31显示出高亲和力组胺H(3)受体。拮抗剂30和31表现出对其他组胺H(1)和H(2)受体亚型的显着选择性,并且在sigma(1)结合测定中具有100倍的选择性。化合物30和31是迄今为止文献中报道的最有效的,选择性的非咪唑组胺H(3)受体拮抗剂。
-
[EN] DIAMINO-PYRIMIDINES AND THEIR USE AS ANGIOGENESIS INHIBITORS<br/>[FR] DIAMINO-PYRIMIDINES ET LEURS UTILISATIONS EN TANT QU'INHIBITEURS DE L'ANGIOGENESE申请人:SMITHKLINE BEECHAM CORP公开号:WO2003074515A1公开(公告)日:2003-09-12Benzimidazole derivatives of formula (I) , which are useful as TIE-2 and/or VEGFR-2 inhibitors are described herein. The described invention also includes methods of making such benzimidazole derivatives as well as methods of using the same in the treatment of hyperproliferative diseases. (I)
-
Bronchorelaxing compounds申请人:Skogvall Staffan公开号:US20050165004A1公开(公告)日:2005-07-28A compound of the general formula (I) including its pharmaceutically acceptable acid addition salts wherein A is CHR 9 , wherein R 9 is H, C 1 -C 6 alkyl; n is 1-3; B is CHR 10 , wherein R 10 is H, C 1 -C 6 alkyl; m is 1 or 2; D is O or S or optionally NR 16 , wherein R 16 is H, C 1 -C 6 alkyl or C 2 -C 6 acyl; E is CR 11 R 12 or NR 13 , wherein R 11 and R 12 are, independent of each other, H or C 1 -C 6 alkyl, R 13 is H or C 1 -C 6 alkyl; F is C 1 -C 18 alkyl which may be mono- or di-unsaturated and/or substituted, is useful in treating and preventing pulmonary disease characterized by bronchoconstriction. Also disclosed are pharmaceutical compositions comprising the compound and methods for their manufacture.通用式(I)的化合物及其药学上可接受的酸盐包括其中 其中A为CHR 9 ,其中R 9 为H,C 1 -C 6 烷基;n为1-3;B为CHR 10 ,其中R 10 为H,C 1 -C 6 烷基;m为1或2;D为O或S或可选地为NR 16 ,其中R 16 为H,C 1 -C 6 烷基或C 2 -C 6 酰基;E为CR 11 R 12 或NR 13 ,其中R 11 和R 12 独立地为H或C 1 -C 6 烷基,R 13 为H或C 1 -C 6 烷基;F为C 1 -C 18 烷基,可以是单烯或双烯和/或取代的,用于治疗和预防以支气管痉挛为特征的肺部疾病。还公开了包含该化合物的药物组合物及其制备方法。
-
[EN] PYRAZOLE DERIVATIVES AS PROTEIN KINASE MODULATORS<br/>[FR] DERIVES DE PYRAZOLE SERVANT DE MODULATEURS DE PROTEINE KINASE申请人:ASTEX TECHNOLOGY LTD公开号:WO2005061463A1公开(公告)日:2005-07-07The invention provides compounds of the formula: (I) having protein kinase B inhibiting activity: wherein A is a saturated hydrocarbon linker group containing from 1 to 7 carbon atoms, the linker group having a maximum chain length of 5 atoms extending between Rl and NR2R3 and a maximum chain length of 4 atoms extending between E and NR2R3, wherein one of the carbon atoms in the linker group may optionally be replaced by an oxygen or nitrogen atom; and wherein the carbon atoms of the inker group A may optionally bear one or more substituents selected from oxo, fluorine and hydroxy, provided that the hydroxy group when present is not located at a carbon atom a with respect to the NR2R3 group and provided that the oxo group when present is located at a carbon atom a with respect to the NR2R3 group; E is a monocyclic or bicyclic carbocyclic or heterocyclic group; Rl is an aryl or heteroaryl group; and R2, R3, R4 and R5 are as defined in the claims. Also provided are pharmaceutical compositions containing the compounds, methods for preparing the compounds and their use as anticancer agents.该发明提供了具有蛋白激酶B抑制活性的化合物,其化学式为:(I),其中A是一个饱和的含有1至7个碳原子的烷基链连接基团,该连接基团在R1和NR2R3之间延伸的最大链长为5个原子,在E和NR2R3之间延伸的最大链长为4个原子,其中连接基团中的一个碳原子可以选择性地被氧原子或氮原子取代;连接基团A的碳原子可以选择性地携带来自酮基、氟和羟基的一个或多个取代基,前提是当存在羟基时,该羟基不位于相对于NR2R3基团的碳原子a处,且当存在酮基时,该酮基位于相对于NR2R3基团的碳原子a处;E是一个单环或双环的碳环或杂环基团;R1是芳基或杂芳基团;R2、R3、R4和R5如权利要求中所定义。还提供了含有这些化合物的药物组合物,制备这些化合物的方法以及它们作为抗癌剂的用途。
表征谱图
-
氢谱1HNMR
-
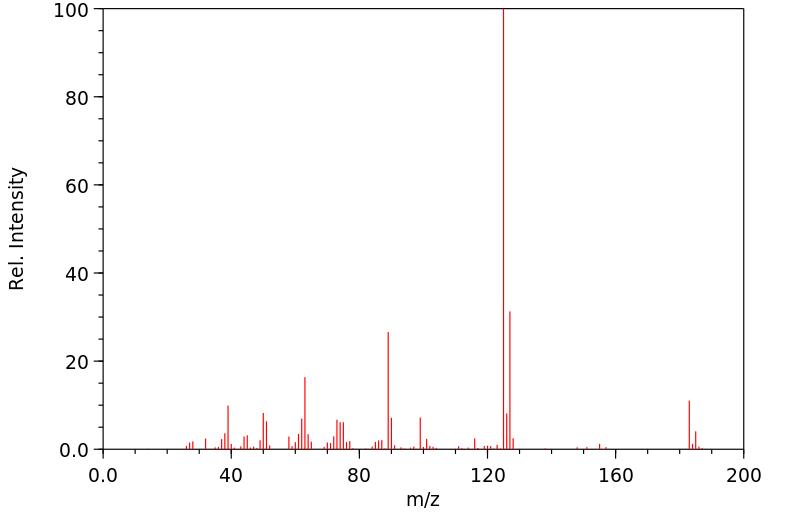
质谱MS
-
碳谱13CNMR
-
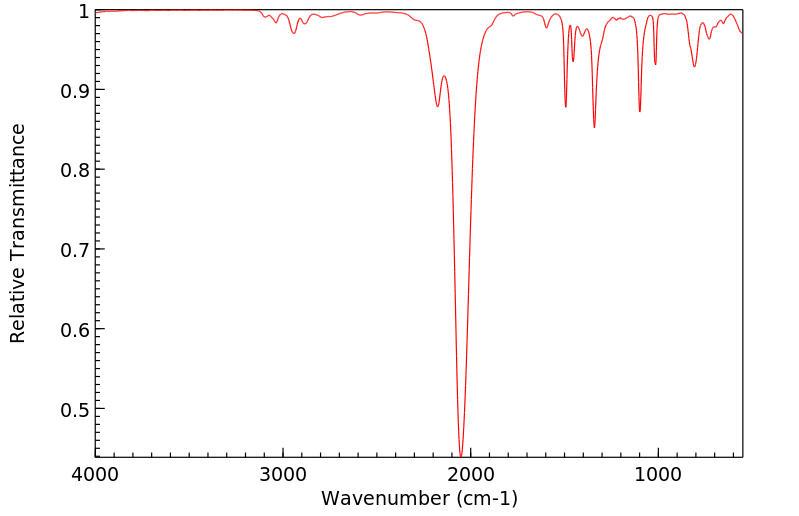
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫