1,4-双(2-羟基乙基)哌嗪 | 122-96-3
中文名称
1,4-双(2-羟基乙基)哌嗪
中文别名
N,N'-Bis(2-羟基乙基)哌嗪;1,4-双(2-羟氧基)对二氮乙环乙烷;N,N'-二羟乙基哌嗪;N,N'-双(2-羟乙基)哌嗪;1,4-双(2-羟乙基)哌嗪;1,4-二(2-羟乙基)哌嗪
英文名称
1,4-bis(2-hydroxyethyl)piperazine
英文别名
2,2'-piperazine-1,4-diyl-bis-ethanol;1,4-Piperazinediethanol;2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanol
CAS
122-96-3
化学式
C8H18N2O2
mdl
MFCD00006157
分子量
174.243
InChiKey
VARKIGWTYBUWNT-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:133.5-136 °C(lit.)
-
沸点:215-220 °C50 mm Hg(lit.)
-
密度:1.097
-
闪点:166℃
-
溶解度:可溶于DMSO(少许)、甲醇(少许)
-
LogP:-1.92 at 20℃
-
物理描述:Liquid
计算性质
-
辛醇/水分配系数(LogP):-1.3
-
重原子数:12
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:46.9
-
氢给体数:2
-
氢受体数:4
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
危险类别码:R36/37/38
-
危险品运输编号:OTH
-
WGK Germany:3
-
RTECS号:TL3675000
-
安全说明:S26,S37/39
-
危险性防范说明:P261,P280,P305+P351+P338
-
危险性描述:H302,H315,H319,H332,H335
-
储存条件:室温
SDS
| Name: | N,N'-Bis(2-Hydroxyethyl)piperazine, 99% Material Safety Data Sheet |
| Synonym: | N,N'-Piperazinediethanol. |
| CAS: | 122-96-3 |
Synonym: N,N'-Piperazinediethanol.
SECTION 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 122-96-3 | N,N'-Bis(2-Hydroxyethyl)piperazine | 99% | 204-586-0 |
Risk Phrases: 36/37/38
SECTION 3 - HAZARDS IDENTIFICATION EMERGENCY OVERVIEW Irritating to eyes, respiratory system and skin. Potential Health Effects
Eye:
Causes eye irritation. May cause chemical conjunctivitis.
Skin:
Causes skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
Causes respiratory tract irritation. May be harmful if inhaled.
Chronic:
No information found.
SECTION 4 - FIRST AID MEASURES
Eyes:
Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
SECTION 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
In case of fire, use water, dry chemical, chemical foam, or alcohol-resistant foam.
SECTION 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions. Provide ventilation.
SECTION 7 - HANDLING and STORAGE
Handling:
Minimize dust generation and accumulation. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. Use with adequate ventilation. Wash clothing before reuse.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
SECTION 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low. Exposure Limits CAS# 122-96-3: Personal Protective Equipment
Eyes:
Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
SECTION 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystalline powder
Color: white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 215-220 deg C@50mmH
Freezing/Melting Point: 134-137 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C8H18N2O2
Molecular Weight: 174.1382
SECTION 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Dust generation.
Incompatibilities with Other Materials:
Strong acids, strong oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
SECTION 11 - TOXICOLOGICAL INFORMATION RTECS#: CAS# 122-96-3: TL3675000
LD50/LC50:
CAS# 122-96-3: Oral, rat: LD50 = 3730 uL/kg; Skin, rabbit: LD50 = >10 mL/kg.
Carcinogenicity:
N,N'-Bis(2-Hydroxyethyl)piperazine - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
SECTION 12 - ECOLOGICAL INFORMATION
SECTION 13 - DISPOSAL CONSIDERATIONS Dispose of in a manner consistent with federal, state, and local regulations.
SECTION 14 - TRANSPORT INFORMATION IATA
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
IMO
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
RID/ADR
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing group:
SECTION 15 - REGULATORY INFORMATION European/International Regulations European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S 37/39 Wear suitable gloves and eye/face protection. WGK (Water Danger/Protection) CAS# 122-96-3: No information available. Canada CAS# 122-96-3 is listed on Canada's DSL List. CAS# 122-96-3 is not listed on Canada's Ingredient Disclosure List. US FEDERAL TSCA CAS# 122-96-3 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
MSDS Creation Date: 11/18/1999 Revision #3 Date: 1/28/2004 The information above is believed to be accurate and represents the best information currently available to us. However, we make no warranty of merchantability or any other warranty, express or implied, with respect to such information, and we assume no liability resulting from its use. Users should make their own investigations to determine the suitability of the information for their particular purposes. In no way shall the company be liable for any claims, losses, or damages of any third party or for lost profits or any special, indirect, incidental, consequential or exemplary damages, howsoever arising, even if the company has been advised of the possibility of such damages.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N-羟乙基哌嗪 1-(2-hydroxyethyl)piperazine 103-76-4 C6H14N2O 130.19 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 三乙烯二胺 1,4-diaza-bicyclo[2.2.2]octane 280-57-9 C6H12N2 112.175 —— N,N'-bis(2-ethylaminoethyl)piperazine 1011486-22-8 C12H28N4 228.381 —— N,N'-bis(2-methylaminoethyl)piperazine 176906-03-9 C10H24N4 200.327 —— Essigsaeure-N,N'-bis- -piperazindiester 20727-34-8 C12H22N2O4 258.318 1,4-二(2-氯乙基)哌嗪 1,4-bis-(2-chloro-ethyl)-piperazine 1009-85-4 C8H16Cl2N2 211.134 —— 1,4-di(6,9-dimethyl-1,3-dioxa-6,9-diaza-2-phosphacycloundecan-2-yl)oxyethylpiperazine —— C24H52N6O6P2 582.661 N-甲基哌嗪 1-methyl-piperazine 109-01-3 C5H12N2 100.164
反应信息
-
作为反应物:描述:参考文献:名称:一种催化加氢制备哌嗪或烷基哌嗪的方法摘要:本发明公开了一种催化加氢制备哌嗪或烷基哌嗪的方法,包括:原料二羟乙基哌嗪或/和羟乙基哌嗪在催化剂作用下进行催化加氢反应,后处理得到哌嗪或/和烷基哌嗪;所述的催化剂中,活性成分为Cu、Ni、Co中的一种或多种。本发明采用二羟乙基哌嗪或羟乙基哌嗪为原料,通过催化加氢制备哌嗪或烷基哌嗪,提供了一条全新的哌嗪或烷基哌嗪的合成工艺;同时可通过该方法实现对含有二羟乙基哌嗪或羟乙基哌嗪废液或者副产物的回收利用;另外,通过现有的方法可实现产品中烷基哌嗪的分离,进一步提高了产品的价值,降低了制备成本。公开号:CN104496939B
-
作为产物:参考文献:名称:Composition that can be used as an emulsifying and dispersing surface agent and its production process摘要:以下内容描述如下:一种由单聚、二聚、三聚和/或四聚脂肪酸衍生的酰胺、胺、酯酰胺、酯胺、胺盐和单甘酯混合物组成的配方;一种生产该配方的工艺,其中包括将热聚合的多不饱和植物油与至少一种氨基醇的过量进行转酰胺反应,可以有或没有催化剂的存在;以及该配方的用途,特别是作为乳化剂,可以根据油的性质形成油在水中或水在油中的乳液,作为固体分散剂,或作为液体或乳液中泡沫稳定剂。公开号:US06221920B1
-
作为试剂:描述:亚叶酸 在 1,4-双(2-羟基乙基)哌嗪 、 5,10-methenyltetrahydrofolate synthetase 、 MgATP 、 2-巯基乙醇 作用下, 生成 5,10-methylidyne-5,6,7,8-tetrahydrofolic acid参考文献:名称:鸡肝中5,10-甲基四氢叶酸合成酶的纯化与鉴定摘要:다있었。다。ATP,Kg,MgATP,MgCTP,MgUTP和MgGTP,以及MgATP和其他产品。用四硝基met烷(1-硝基-3-(3-二甲基氨基丙基)-碳二亚胺),酪氨酸和羧酸盐进行脱硫。产品名称:5,10-甲基四氢叶酸合成酶,간,대사,테트라니트로메탄,1-乙基-3-(3-二甲基氨基丙基)-碳二亚胺摘要。通过30-70%硫酸铵分级分离,Q Sepharose Fast Flow阴离子交换和Source 15Phe疏水作用色谱法纯化来自鸡肝的5,10-甲基四氢叶酸合成酶。细胞提取物,硫酸铵,Q Sepharose Fast Flow和Source 15Phe的比活分别为0.0085、0.031、0.80和1.27 U / mg。细胞提取物,硫酸铵,Q Sepharose Fast Flow和Source 15Phe的纯化折叠活性分别为1,分别为3.7、94.1和149.4。HPLC凝胶DOI:10.5012/jkcs.2010.54.5.567
文献信息
-
PHARMACEUTICAL COMPOUNDS AS INHIBITORS OF CELL PROLIFERATION AND THE USE THEREOF申请人:ANDERSON MARK B.公开号:US20100068197A1公开(公告)日:2010-03-18Disclosed are compounds of Formula I effective as cytotoxic agents. The compounds of this invention are useful in the treatment of a variety of clinical conditions in which uncontrolled growth and spread of abnormal cells occurs.揭示的是作为细胞毒性剂有效的I式化合物。本发明的化合物在治疗多种临床病况中是有用的,这些病况中发生异常细胞的不受控制的生长和扩散。
-
Iodinated Choline Transport-Targeted Tracers作者:Pavel Švec、Zbyněk Nový、Jan Kučka、Miloš Petřík、Ondřej Sedláček、Martin Kuchař、Barbora Lišková、Martina Medvedíková、Kristýna Kolouchová、Ondřej Groborz、Lenka Loukotová、Rafał Ł. Konefał、Marián Hajdúch、Martin HrubýDOI:10.1021/acs.jmedchem.0c01710日期:2020.12.24theranostics for diseases accompanied by pathological function of proteins involved in choline transport. Unlike choline analogues labeled with 11C or 18F that are currently used in the clinic, the iodinated compounds described herein are applicable in positron emission tomography, single-photon emission computed tomography, and potentially in therapy, depending on the iodine isotope selection. Moreover
-
[EN] ANTIOXIDANT CAMPTOTHECIN DERIVATIVES AND ANTIOXIDANT ANTINEOPLASTIC NANOSPHERES THEREOF<br/>[FR] DÉRIVÉS ANTIOXYDANTS DE LA CAMPTOTHÉCINE ET LEURS NANOSPHÈRES ANTIOXYDANTES ANTINÉOPLASIQUES申请人:CEDARS SINAI MEDICAL CENTER公开号:WO2010060098A1公开(公告)日:2010-05-27The present invention is directed to antioxidant derivatives of camptothecin and antioxidant derivatives of camptothecin analogs and the preparation of nanometer-sized camptothecin prodrugs. Methods of synthesizing the antioxidant derivatives of camptothecin and antioxidant derivatives of camptothecin analogs, spontaneous emulsification or nanoprecipitation thereof to produce antioxidant camptothecin nanosphere prodrugs and their use in treating cancerous diseases are also provided. A further aspect of this invention is the use of these antioxidant camptothecin nanosphere prodrugs for the preparation of delivery devices of other pharmaceuticals and/or drugs.
-
[EN] 2, 3, 6-TRISUBSTITUTED-4-PYRIMIDONE DERIVATIVES<br/>[FR] DERIVES DE 2,3,6-TRISUBSTITUE 4-PYRIMIDONE申请人:MITSUBISHI PHARMA CORP公开号:WO2004085408A1公开(公告)日:2004-10-07A pyrimidone derivative having tau protein kinase 1 inhibitory activity which is represented by formula (I) or a salt thereof, or a solvate thereof or a hydrate thereof; useful for prventive and/or therapeutic treatment of diseass such as neurodegenerative diseases (e.g. Alzheimer disease); wherein Q represents CH or nitrogen atom; R represents a C1-C12 alkyl group; the ring of Formula (I): represents piperazine ring or piperidine ring; each X independently represents a C1-C8 alkyl group, an optionally partially hydrogenated C6-C10 aryl ring, an indan ring or the like; m represents an integer of 1 to 3; each Y independently represents a halogen atom, a hydroxy group, a cyano group, a C1-C6 alkyl group or the like; n represents an integer of 0 to 8; when X and Y or two Y groups are attached on the same carbon atom, they may combine to each other to form a C2-C6 alkylene group.
-
PROCESS FOR PREPARING DI-, TRI- AND POLYAMINES BY HOMOGENEOUSLY CATALYZED ALCOHOL AMINATION申请人:SCHAUB Thomas公开号:US20120232293A1公开(公告)日:2012-09-13Process for preparing primary amines which have at least one functional group of the formula (—CH 2 —NH 2 ) and at least one further primary amino group by alcohol amination of starting materials having at least one functional group of the formula (—CH 2 —OH) and at least one further functional group (—X), where (—X) is selected from among hydroxyl groups and primary amino groups, by means of ammonia with elimination of water, wherein the reaction is carried out homogeneously catalyzed in the presence of at least one complex catalyst comprising at least one element selected from groups 8, 9 and 10 of the Periodic Table and also at least one donor ligand.
表征谱图
-
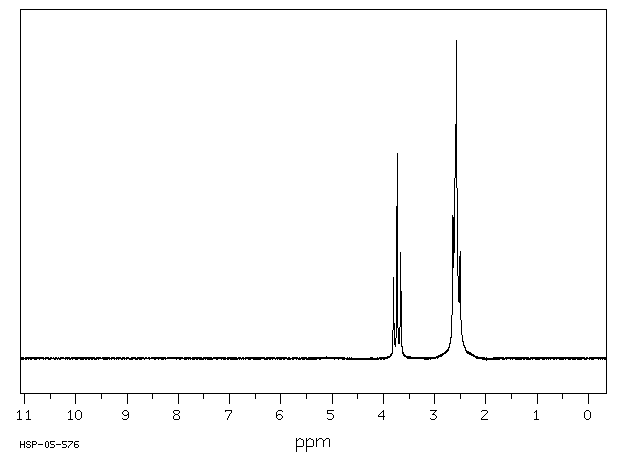
氢谱1HNMR
-
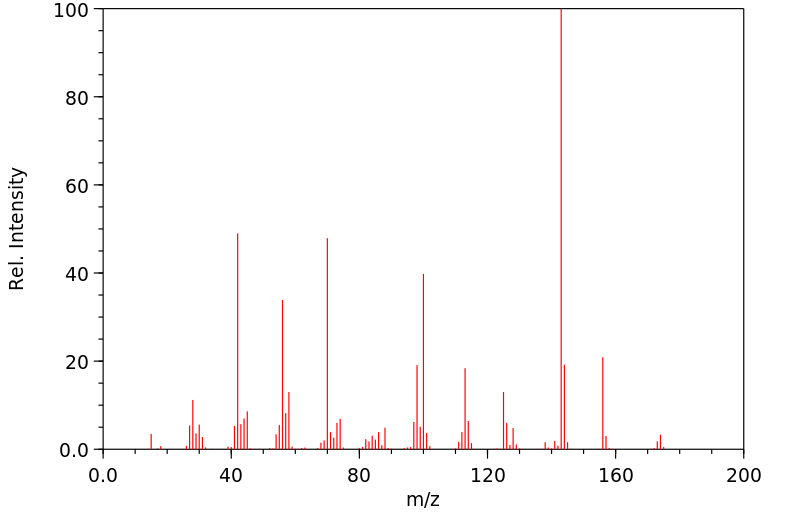
质谱MS
-
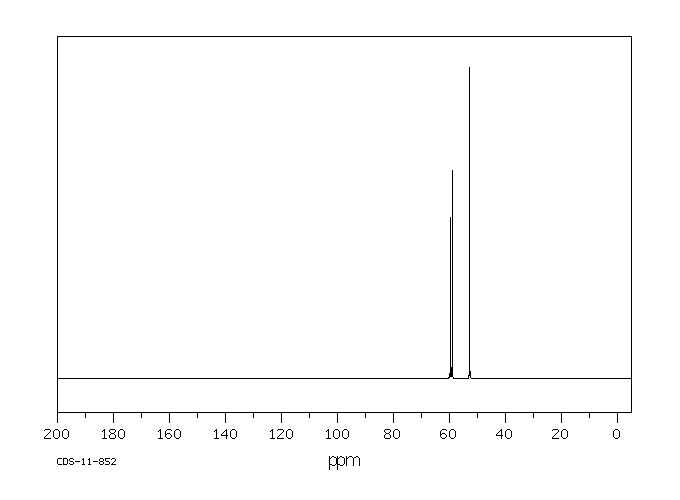
碳谱13CNMR
-
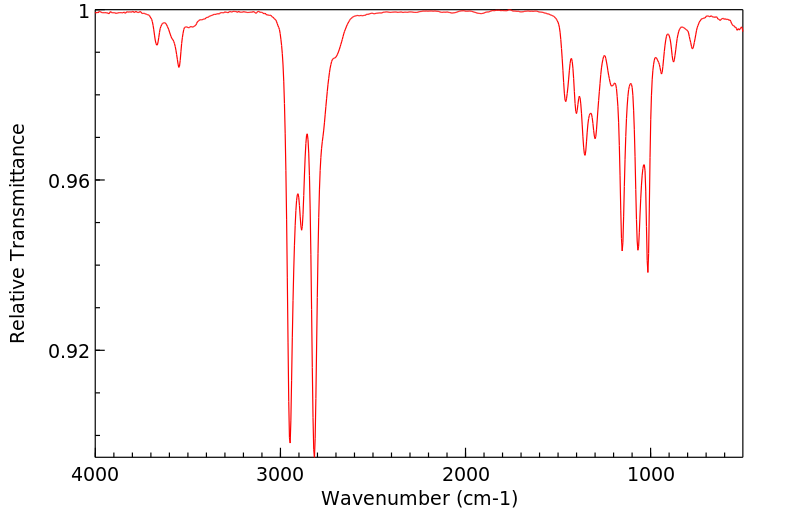
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2S)-4-[7-(8-氯-1-萘)-5,6,7,8-四氢-2-[[((2S)-1-甲基-2-吡咯烷基]甲氧基]吡啶基[3,4-d]嘧啶-4-基]-1-(2-氟-1-氧代-2-丙烯-1-基)-2-哌Chemicalbook嗪乙腈;2-((S)-4-(7-(8-氯萘-1-基)-2-((((S)-1-
齐拉西酮相关物质C
齐拉西酮杂质E
齐拉西酮开环物,氨基酸杂质
齐拉西酮亚砜
齐拉西酮 盐酸盐 一水合物
齐拉西酮
鲸蜡硬脂醇
鲁拉西酮杂质3
鲁拉西酮杂质23
鲁拉西酮杂质14
鲁拉西酮杂质11
鲁拉西酮
鲁拉西杂质E
鲁拉西杂质1
高分子量聚合三嗪类无卤阻燃剂
驱虫灵D
马福拉嗪
马来酸阿伐曲泊帕
顺式-1-乙酰基-2,6-二甲基-4-亚硝基-哌嗪
雷诺嗪双(N-氧化物)
陶扎色替
阿达色林
阿莫西林二氧代哌嗪
阿立哌唑羟基丁基杂质
阿立哌唑杂质26
阿立哌唑USP相关物质H
阿立哌唑N4-氧化物
阿立哌唑N,N-二氧化物
阿立哌唑EP杂质D
阿立哌唑-d8
阿立哌唑
阿泊替尼
阿替韦啶
阿拉诺丁
阿扎哌醇
阿得巴司
阿尔哌汀
阿伐曲泊帕杂质
间羟基苯基哌嗪
钾 1-甲基-4-三氟硼酸三甲基哌嗪
钠4-(4-乙酰基-1-哌嗪基)苯酚
酮齐拉西酮
酮酮唑油酸酯
酚酞单磷酸酯二-(2-氨基-2-甲基-1,3-丙二醇)盐
选择性氟试剂II
达鲁舍替
达哌唑
赫普索
赤霉素A7甲酯