2-乙基噻唑烷 | 24050-09-7
中文名称
2-乙基噻唑烷
中文别名
——
英文名称
2-ethylthiazolidine
英文别名
2-ethyl-thiazolidine;2-Aethyl-thiazolidin;2-ethyl-1,3-thiazolidine
CAS
24050-09-7
化学式
C5H11NS
mdl
——
分子量
117.215
InChiKey
SZLVYXIKAAFTFY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:170-175 °C
-
密度:1.0216 g/cm3
-
LogP:-0.680 (est)
-
保留指数:991;983
计算性质
-
辛醇/水分配系数(LogP):1.3
-
重原子数:7
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
海关编码:2934999090
SDS
反应信息
-
作为反应物:描述:2-乙基噻唑烷 在 盐酸 、 水 、 O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate 、 溶剂黄146 、 N,N-二异丙基乙胺 作用下, 以 四氢呋喃 、 甲醇 、 二氯甲烷 、 N,N-二甲基甲酰胺 为溶剂, 反应 8.0h, 生成 methyl 1-(4-(6-((S)-2-ethylthiazolidine-3-carbonyl)benzo[d]thiazol-2-yl)-3-fluorobenzyl)azetidine-3-carboxylate参考文献:名称:Novel 5- and 6-subtituted benzothiazoles with improved physicochemical properties: Potent S1P1 agonists with in vivo lymphocyte-depleting activity摘要:An SAR campaign designed to increase polarity in the 'tail' region of benzothiazole 1 resulted in two series of structurally novel 5- and 6-substituted S1P(1) agonists. Structural optimization for potency ultimately delivered carboxamide (+)-11f, which in addition to possessing improved physicochemical properties relative to starting benzothiazole 1, also displayed good S1P(3) selectivity and acceptable in vivo lymphocyte-depleting activity. (C) 2011 Published by Elsevier Ltd.DOI:10.1016/j.bmcl.2011.10.069
-
作为产物:参考文献:名称:Ratschinskii et al., Zhurnal Obshchei Khimii, 1958, vol. 28, p. 2998,3001,3002;engl.Ausg.S.3027,3028,3030摘要:DOI:
文献信息
-
Mechanistic Studies on Thiazolidine Formation in Aldehyde/Cysteamine Model Systems作者:Tzou-Chi Huang、Lee-Zen Huang、Chi-Tang HoDOI:10.1021/jf9705633日期:1998.1.1A mechanism was proposed to elucidate the formation of a thiazolidine in aldehyde/cysteamine model systems. Buffer dramatically promotes thiazolidine formation from formaldehyde and cysteamine. Phosphate tends to stabilize the primary carbocation formed, and this may lead to completion of the cyclization by attack of the amino nitrogen on the activated carbon. Protic solvent, by removing the water
-
Yasuhara, Akio; Shibamoto, Takayuki, Agricultural and Biological Chemistry, 1989, vol. 53, # 8, p. 2273 - 2274作者:Yasuhara, Akio、Shibamoto, TakayukiDOI:——日期:——
-
Okumura, Joji, Agricultural and Biological Chemistry, 1991, vol. 55, # 6, p. 1537 - 1545作者:Okumura, JojiDOI:——日期:——
-
YASUHARA, AKIO;SHIBAMOTO, TAKAYUKI, AGR. AND BIOL. CHEM., 53,(1989) N, C. 2273-2274作者:YASUHARA, AKIO、SHIBAMOTO, TAKAYUKIDOI:——日期:——
-
BALKO, T. W.;HACKLER, R. E.作者:BALKO, T. W.、HACKLER, R. E.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
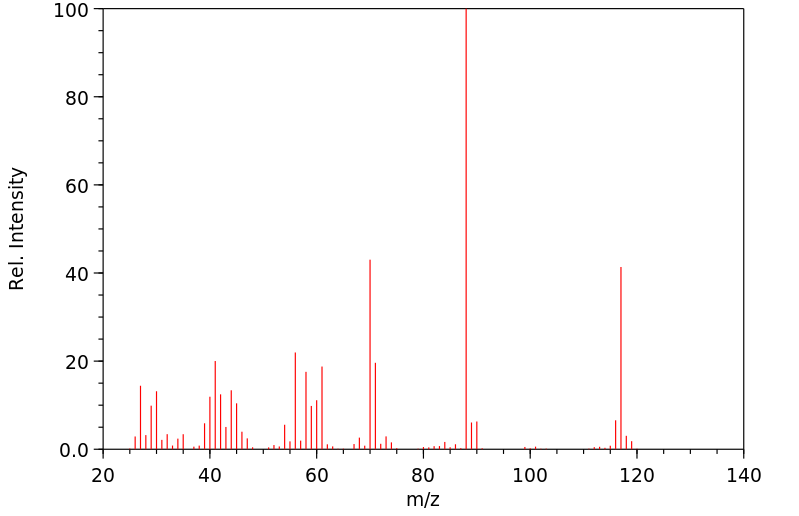
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-4-异丙基-2-恶唑烷硫酮
麻黄恶碱
顺-八氢-2H-苯并咪唑-2-酮
顺-1-(4-氟苯基)-4-[1-(4-氟苯基)-4-羰基-1,3,8-三氮杂螺[4.5]癸-8-基]环己甲腈
非达司他
降冰片烯缩醛3-((1S,2S,4S)-双环[2.2.1]庚-5-烯-2-羰基)恶唑烷-2-酮
阿齐利特
阿那昔酮
阿洛双酮
阿帕鲁胺
阿帕他胺杂质2
铟烷-2-YL-甲基胺盐酸
钾3-{2-[3-氰基-3-(十二烷基磺酰基)-2-丙烯-1-亚基]-1,3-噻唑烷-3-基}-1-丙烷磺酸酯
钠2-{[4,5-二羟基-3-(羟基甲基)-2-氧代-1-咪唑烷基]甲氧基}乙烷磺酸酯
重氮烷基脲
詹氏催化剂
解草恶唑
解草噁唑
表告依春
螺莫司汀
螺立林
螺海因氮丙啶
螺[咪唑烷-4,3'-吲哚啉]-2,2',5-三酮
螺[1-氮杂双环[2.2.2]辛烷-8,5'-咪唑烷]-2',4'-二酮
苯甲酸,4-氟-,2-[5,7-二(三氟甲基)-1,8-二氮杂萘-2-基]-2-甲基酰肼
苯氰二硫酸,1-氰基-1-甲基-4-氧代-4-(2-硫代-3-噻唑烷基)丁酯
苯妥英钠杂质8
苯妥英钠
苯妥英-D10
苯妥英
苯基硫代海因半胱氨酸钠盐
苯基硫代乙内酰脲-谷氨酸
苯基硫代乙内酰脲-蛋氨酸
苯基硫代乙内酰脲-苯丙氨酸
苯基硫代乙内酰脲-色氨酸
苯基硫代乙内酰脲-脯氨酸
苯基硫代乙内酰脲-缬氨酸
苯基硫代乙内酰脲-异亮氨酸
苯基硫代乙内酰脲-天冬氨酸
苯基硫代乙内酰脲-亮氨酸
苯基硫代乙内酰脲-丙氨酸
苯基硫代乙内酰脲-D-苏氨酸
苯基硫代乙内酰脲-(NΕ-苯基硫代氨基甲酰)-赖氨酸
苯基乙内酰脲-甘氨酸
苏氨酸-1-(苯基硫基)-2,4-咪唑烷二酮(1:1)
色氨酸标准品002
膦酸,(2-羰基-1-咪唑烷基)-,二(1-甲基乙基)酯
脱氢-1,3-二甲基尿囊素
脱氢-1,3,8-三甲基尿囊素
聚(d(A-T)铯)