4-氨基-N,N-二乙基苯磺酰胺 | 1709-39-3
中文名称
4-氨基-N,N-二乙基苯磺酰胺
中文别名
N1-二乙基磺胺;N'-二乙基磺胺
英文名称
4-(N,N-diethylaminosulfonyl)aniline
英文别名
4-Amino-N,N-diethyl-benzenesulfonamide;N1,N1-Diethyl-sulfanilamid;NSC 15769;4-Amino-N,N-diethylbenzenesulfonamide
CAS
1709-39-3
化学式
C10H16N2O2S
mdl
MFCD01055489
分子量
228.315
InChiKey
LTFVELCIFWEGGA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:105-106 °C
-
沸点:375.9±44.0 °C(Predicted)
-
密度:1.199±0.06 g/cm3(Predicted)
-
溶解度:>34.2 [ug/mL]
计算性质
-
辛醇/水分配系数(LogP):1.1
-
重原子数:15
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:71.8
-
氢给体数:1
-
氢受体数:4
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
海关编码:2935009090
-
危险类别:IRRITANT
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: N,N-Diethyl 4-aminobenzenesulfonamide
Synonyms: 4-Amino-N,N-diethylbenzenesulfonamide
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: N,N-Diethyl 4-aminobenzenesulfonamide
CAS number: 1709-39-3
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C10H16N2O2S
Molecular weight: 228.3
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, sulfur oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: N,N-Diethyl 4-aminobenzenesulfonamide
Synonyms: 4-Amino-N,N-diethylbenzenesulfonamide
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: N,N-Diethyl 4-aminobenzenesulfonamide
CAS number: 1709-39-3
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C10H16N2O2S
Molecular weight: 228.3
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, sulfur oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N-[4-(二乙基氨基磺酰基)苯基]乙酰胺 N4-acetyl-N1-diethylsulfanilamide 2080-32-2 C12H18N2O3S 270.353 N,N-二乙基4-硝基苯磺酰胺 N,N-Diethyl-4-nitro-benzenesulfonamide 89840-82-4 C10H14N2O4S 258.298 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— N,N-diethyl-4-methylaminobenzenesulfonamide 1094225-40-7 C11H18N2O2S 242.342 —— N,N-diethyl-4-isothiocyanatobenzenesulfonamide 99841-31-3 C11H14N2O2S2 270.376 —— N-[4-(Diethylsulfamoyl)phenyl]glycine 88327-97-3 C12H18N2O4S 286.352 —— Ethyl 2-chloro-2-[[4-(diethylsulfamoyl)phenyl]hydrazinylidene]acetate 96722-51-9 C14H20ClN3O4S 361.849 —— Phenyl [4-(diethylsulfamoyl)phenyl]carbamate 88429-01-0 C17H20N2O4S 348.423 —— N-(N-acetyl-sulfanilyl)-sulfanilic acid diethylamide 315672-03-8 C18H23N3O5S2 425.53 —— {[4-(Diethylsulfamoyl)phenyl](nitroso)amino}acetic acid 88328-03-4 C12H17N3O5S 315.35 —— Diethyl 2-[[4-(diethylsulfamoyl)anilino]methylidene]propanedioate —— C18H26N2O6S 398.48 —— 4-[N-(1-benzyl-piperidin-4-yl)-amino]-N,N-diethyl benzenesulfonamide —— C22H31N3O2S 401.573 —— N-[4-(N,N-Diethylaminosulfonyl)phenyl]-3,3,3-trifluoro-2-hydroxy-2-methylpropanamide —— C14H19F3N2O4S 368.377 —— N-(4-Diethylsulfamoyl-phenyl)-2,2-diphenyl-acetamide —— C24H26N2O3S 422.548 - 1
- 2
反应信息
-
作为反应物:描述:4-氨基-N,N-二乙基苯磺酰胺 以 乙醇 为溶剂, 反应 1.33h, 生成 3-(4-N,N-diethylaminosulphonephenylamino)-1-phenylpyrazolo[3,4-d]pyrimidine-4,6-dione参考文献:名称:乙烯酮二硫缩醛的杂芳化:第二部分。一些新型 5-氨基吡唑-3-甲腈、3-甲酰胺和吡唑并[3,4-d]嘧啶-4-酮衍生物作为抗菌剂的合成摘要:摘要 乙烯酮二硫代缩醛 1a-d 用于合成吡唑二硫代羧酸酯 2a-d 和芳基氨基吡唑 4a、b,在芳基残基中具有两个不同的取代基(邻乙氧基和对二乙基氨基磺酰基)。描述了二硫酯 2 ate 的一些转化。之后,芳基氨基吡咯4a,b 被转化为吡唑并嘧啶衍生物8-11。DOI:10.1080/10426500108040260
-
作为产物:参考文献:名称:基于降冰片基的碳环核苷类似物作为细胞周期蛋白依赖性激酶 2 抑制剂的优化。摘要:我们报告了降冰片基部分作为细胞周期蛋白依赖性激酶 2 (CDK2) 抑制剂的新型结构基序的发现,该基序是通过筛选碳环核苷类似物文库确定的。通过使用药物化学方法将三个微摩尔命中扩展为一系列 16 种新化合物。它们对 CDK2 具有显着的微摩尔活性,并且该系列中最好的化合物达到了 IC 50190 纳米。通过建模和对接在分子细节中探索结合模式。使用基于量子力学的评分来合理化亲和力。总之,所发现的 9-羟甲基降冰片基部分通过联合实验-理论努力表明能够作为 CDK2 抑制剂的新取代基。这一发现为探索化学空间以寻找更有效的靶向这一类重要蛋白激酶的衍生物打开了大门。DOI:10.1002/jmr.2842
文献信息
-
Fused heterocyclic compounds useful as kinase modulators申请人:Vaccaro Wayne公开号:US20070078136A1公开(公告)日:2007-04-05Compounds having the formula (1), and enantiomers, and diastereomers, pharmaceutically-acceptable salts, thereof, are usefuil as kinase modulators, including MK2 modulation, wherein one of E and F is a nitrogen atom and the other of E and F is a carbon atom, Z is N or CR 3 , and R 1 , R 2 , R 3 , X and Y are as defined herein.具有化学式(1)的化合物,以及其对映体、非对映体、药用可接受的盐,可用作激酶调节剂,包括MK2调节,其中E和F中的一个是氮原子,另一个是碳原子,Z是N或CR3,R1、R2、R3、X和Y如本文所定义。
-
Optimization of a urea-containing series of nicotinamide phosphoribosyltransferase (NAMPT) activators作者:Anthony B. Pinkerton、E. Hampton Sessions、Paul Hershberger、Patrick R. Maloney、Satyamaheshwar Peddibhotla、Meghan Hopf、Eduard Sergienko、Chen-Ting Ma、Layton H. Smith、Michael R. Jackson、Jun Tanaka、Takashi Tsuji、Mayuko Akiu、Steven E. Cohen、Tsuyoshi Nakamura、Stephen J. GardellDOI:10.1016/j.bmcl.2021.128007日期:2021.6neurodegeneration, and muscle wasting disorders. A novel strategy to boost NAD+ is to activate nicotinamide phosphoribosyltransferase (NAMPT), the putative rate-limiting step in the NAD+ salvage pathway. We previously showed that NAMPT activators increase NAD+ levels in vitro and in vivo. Herein we describe the optimization of our NAMPT activator prototype (SBI-0797812) leading to the identification of 1-(4-((4-ch
-
An Old Story in the Parallel Synthesis World: An Approach to Hydantoin Libraries作者:Andrey V. Bogolubsky、Yurii S. Moroz、Olena Savych、Sergey Pipko、Angelika Konovets、Maxim O. Platonov、Oleksandr V. Vasylchenko、Vasyl V. Hurmach、Oleksandr O. GrygorenkoDOI:10.1021/acscombsci.7b00163日期:2018.1.8An approach to the parallel synthesis of hydantoin libraries by reaction of in situ generated 2,2,2-trifluoroethylcarbamates and α-amino esters was developed. To demonstrate utility of the method, a library of 1158 hydantoins designed according to the lead-likeness criteria (MW 200–350, cLogP 1–3) was prepared. The success rate of the method was analyzed as a function of physicochemical parameters
-
Mesoionic Carbene–Iridium Complex Catalyzed <i>Ortho</i>-Selective Hydrogen Isotope Exchange of Anilines with High Functional Group Tolerance作者:Wei Liu、Lei Cao、Zengyu Zhang、Gang Zhang、Shiqing Huang、Linwei Huang、Peng Zhao、Xiaoyu YanDOI:10.1021/acs.orglett.0c00402日期:2020.3.20Mesoionic carbene-iridium complex 1a has been investigated in the hydrogen isotope exchange (HIE) reaction of anilines. By employing 1 mol % of 1a as catalyst, anilines were selectively deuterated at the ortho-position with high deuteration levels. High ortho-selectivity was observed for anilines with various competing directing groups, which is in contrast with catalytic results of Kerr's catalysts
-
A simple synthesis of anilines by LiAlH4/TiCl4 reduction of aromatic nitro compounds作者:Maria Luisa Di Gioia、Antonella Leggio、Isabella Federica Guarino、Vanessa Leotta、Emanuela Romio、Angelo LiguoriDOI:10.1016/j.tetlet.2015.07.089日期:2015.9A rapid and efficient single-step synthesis of substituted anilines has been developed. The aromatic nitro compounds were reduced by using reducing systems generated by the action of an excess of LiAlH4 on TiCl4. Anilines substituted with different functional groups were synthesized in high yields and purity starting from the corresponding nitro compounds. The developed procedure is applicable to nitroaromatics
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
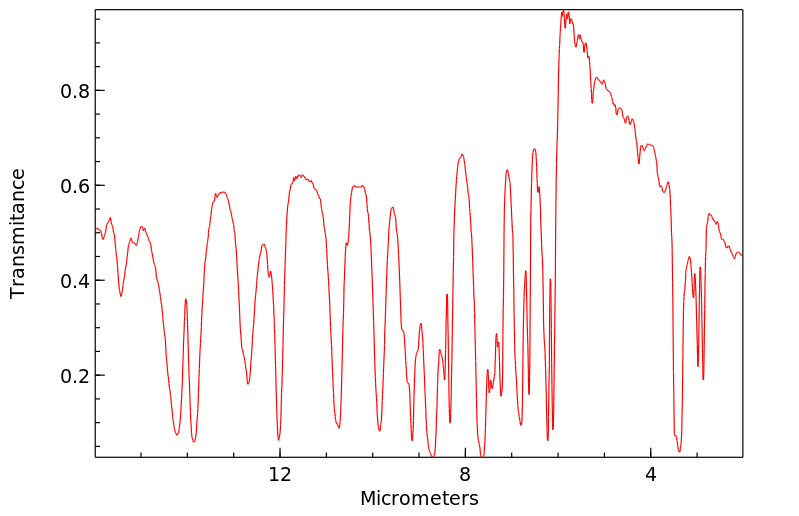
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫