2-甲基丁基异硫氰氰酸酯 | 4404-51-7
中文名称
2-甲基丁基异硫氰氰酸酯
中文别名
2-甲基丁基硫代异氰酸酯
英文名称
1-isothiocyanato-2-methylbutane
英文别名
(2-methylbutyl) isothiocyanate;2-methylbutyl isothiocyanate;2-mehylbutyl isothiocyanate;2-Methyl-butyl-isothiocyanat
CAS
4404-51-7
化学式
C6H11NS
mdl
——
分子量
129.226
InChiKey
MKWQGOSIKKPMLW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:84 °C
-
密度:0.95
-
闪点:84-86°C/15mm
-
LogP:2.880 (est)
-
保留指数:1412
-
稳定性/保质期:
在常温常压下保持稳定
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:8
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.83
-
拓扑面积:44.4
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险等级:6.1
-
危险类别码:R23/24/25
-
危险品运输编号:UN 2810
-
海关编码:2930909090
-
包装等级:III
-
危险类别:6.1
-
安全说明:S36,S37,S39,S45
-
储存条件:请将药品存放在避光、通风干燥的地方,并密封保存。
SDS
| Name: | 2-Methylbutyl isothiocyanate Material Safety Data Sheet |
| Synonym: | |
| CAS: | 4404-51-7 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 4404-51-7 | 2-Methylbutyl isothiocyanate | 95+% | unlisted |
Risk Phrases: 23/24/25 34
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Toxic by inhalation, in contact with skin and if swallowed. Causes burns.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin burns. Toxic in contact with skin.
Ingestion:
Causes gastrointestinal tract burns. Toxic if swallowed.
Inhalation:
Causes respiratory tract irritation. Toxic if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid immediately. Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid immediately. Wash mouth out with water.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container.
Section 7 - HANDLING and STORAGE
Handling:
Do not breathe dust, vapor, mist, or gas. Do not get in eyes, on skin, or on clothing. Use only in a chemical fume hood.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 4404-51-7: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: Pale yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 84 - 86 deg C @15mmHg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C6H11NS
Molecular Weight: 129.23
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents, strong bases, amines, reducing agents.
Hazardous Decomposition Products:
Carbon monoxide, oxides of nitrogen, oxides of sulfur, carbon dioxide.
Hazardous Polymerization: Not available.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 4404-51-7 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2-Methylbutyl isothiocyanate - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: TOXIC LIQUID, CORROSIVE, ORGANIC, N.O.S.*
Hazard Class: 6.1 (8)
UN Number: 2927
Packing Group: II
IMO
Shipping Name: TOXIC LIQUID, CORROSIVE, ORGANIC, N.O.S.
Hazard Class: 6.1 (8)
UN Number: 2927
Packing Group: II
RID/ADR
Shipping Name: TOXIC LIQUID, CORROSIVE, ORGANIC, N.O.S.
Hazard Class: 6.1 (8)
UN Number: 2927
Packing group: II
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: T
Risk Phrases:
R 23/24/25 Toxic by inhalation, in contact with skin
and if swallowed.
R 34 Causes burns.
Safety Phrases:
S 23 Do not inhale gas/fumes/vapour/spray.
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 36/37/39 Wear suitable protective clothing, gloves
and eye/face protection.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 4404-51-7: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 4404-51-7 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 4404-51-7 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:2-甲基丁基异硫氰氰酸酯 在 吡啶 、 potassium carbonate 、 potassium hydroxide 、 sodium hydroxide 作用下, 以 乙醇 、 二氯甲烷 、 水 、 N,N-二甲基甲酰胺 、 正丁醇 为溶剂, 反应 17.0h, 生成 [(5Z,5'Z)-2,2'-(ethane-1,2-diyldisulfanyldiyl)bis(3-(2-methylbutyl)-5-(2-pyridylmethylidene)-3,5-dihydro-4H-imidazol-4-one)]dicopper(II) tetrachloride参考文献:名称:双齿含氮配体双-5-(2-吡啶基亚甲基)-3,5-二氢-4H-咪唑-4-酮的双核铜(II)配合物的合成,表征和细胞毒性摘要:摘要处理含两个5-(2-吡啶基亚甲基)-2-硫代-3,5-二氢-4H-咪唑-4-酮片段的N4-型有机配体的方法是通过硫原子之间的(CH2)2桥连接CuCl 2提供相应配体的双核铜(II)配合物。分离出一系列具有L(CuCl2)2组成的铜配合物,并通过元素分析,UV-Vis光谱和循环伏安法进行了表征。四种合成的配合物的X射线晶体结构均显示出两个铜原子的扭曲的四面体环境。Cu-Cu的距离约为4.5 A,这证实了铜离子之间不存在相互作用。配合物的电化学研究表明,每个配位的铜原子在+0.39至+0.43 V的电位下同时还原。DOI:10.1016/j.poly.2018.04.005
-
作为产物:参考文献:名称:通过氨基甲酰叠氮化物的光催化活化获得环状 N-三氟甲基脲摘要:我们报告了使用蓝光/[Ir] 催化的策略将氨基甲酰基叠氮化物温和活化为相应的氮烯,这使得立体定向获得N-三氟甲基咪唑啉酮和苯并咪唑酮成为可能。这些新颖的结构基序被证明是高度稳健的,允许它们的下游多样化。在我们结合计算和实验研究的基础上,我们建议经历电子与激发的金属催化剂的反弹,包括还原引发的氮损失,然后氧化成相应的氨基甲酰基氮烯和随后的 C-H 插入。DOI:10.1021/jacs.2c02004
文献信息
-
[EN] SUBSTITUTED (PYRIDYL)-AZINYLAMINE DERIVATIVES AS PLANT PROTECTION AGENTS<br/>[FR] DÉRIVÉS SUBSTITUÉS DE (PYRIDYL)-AZINYLAMINE AU TITRE D'AGENTS DE PROTECTION DE VÉGÉTAUX申请人:BAYER CROPSCIENCE SA公开号:WO2010055114A1公开(公告)日:2010-05-20The present invention relates (pyridyl)-azinylamino derivatives of Formula (I), wherein Q1 and p, Ra to Rc, X, Y, Z, L2 and Q2 represent various substituents, their process of preparation, preparation intermediate compounds, their use as fungicide active agents, particularly in the form of fungicide compositions, and methods for the control of phytopathogenic fungi, notably of plants, using these compounds or compositions.
-
[EN] USE OF DERIVATIVES OF 2, 4-DIHYDRO-[1,2,4]TRIAZOLE-3-THIONE AS INHIBITORS O FTEH ENZYME MYELOPEROXIDASE (MPO)<br/>[FR] UTILISATION DE DERIVES DE 2, 4-DIHYDRO-[1,2,4]TRIAZOLE-3-THIONES COMME INHIBITEURS DE L'ENZYME MYELOPEROXYDASE (MPO)申请人:ASTRAZENECA AB公开号:WO2004096781A1公开(公告)日:2004-11-11There is disclosed the use of a compound of formula (I) wherein X, Y, W and Q are as defined in the specification, and pharmaceutically acceptable salts thereof, in the manufacture of a medicament, for the treatment or prophylaxis of diseases or conditions in which inhibition of the enzyme myeloperoxidase (MPO) is beneficial. Certain novel compounds of formula (I) and pharmaceutically acceptable salts thereof are disclosed, together with processes for their preparation. The compounds of formulae (I) are MPO inhibitors and are thereby particularly useful in the treatment or prophylaxis of neuroinflammatory disorders.
-
[EN] FUSED 1,2,4-THIADIAZINE DERIVATIVES, THEIR PREPARATION AND USE<br/>[FR] DERIVES DE 1,2,4-THIADIAZINE FUSIONNEE, LEUR PREPARATION ET UTILISATION申请人:NOVO NORDISK A/S公开号:WO1999003861A1公开(公告)日:1999-01-28(EN) 1,2,4-Thiadiazine and 1,4-thiazine derivates represented by formula (I) wherein A, B, D, R1, R2, R3 and R4 are defined in the description, compositions thereof and methods for preparing the compounds are described. The compounds are useful in the treatment of diseases of the central nervous system, the cardiovascular system, the pulmonary system, the gastrointestinal system and the endocrinological system.(FR) L'invention concerne des dérivés de 1,2,4-thiadiazine et de 1,4-thiazine représentés par la formule (I), dans laquelle A, B, D, R1, R2, R3 et R4 sont tels que définis dans le descriptif. L'invention concerne en outre des compositions de ces dérivés et des procédés de préparation des composés. Les composés sont utiles dans le traitement de maladies du système nerveux central, l'appareil cardio-vasculaire, le système pulmonaire, l'appareil digestif et le système endocrinien.
-
Fused 1,2,4-thiadiazine derivatives, their preparation and use申请人:Novo Nordisk A/S公开号:US06225310B1公开(公告)日:2001-05-011,2,4-Thiadiazine and 1,4-thiazine derivates represented by formula (I): wherein A, B, D, R1, R2, R3 and R4 are defined in the description, compositions thereof and methods for preparing the compounds are described. The compounds are useful in the treatment of diseases of the central nervous system, the cardiovascular system, the pulmonary system, the gastrointestinal system and the endocrinological system.
-
Use of derivatives of 2, 4-dihydro-[1,2,4] triazole-3-thione as inhibitors of the enzyme myeloperoxidase (mpo)申请人:Svensson Mats公开号:US20070093483A1公开(公告)日:2007-04-26There is disclosed the use of a compound of formula (I) wherein X, Y, W and Q are as defined in the specification, and pharmaceutically acceptable salts thereof, in the manufacture of a medicament, for the treatment or prophylaxis of diseases or conditions in which inhibition of the enzyme myeloperoxidase (MPO) is beneficial. Certain novel compounds of formula (I) and pharmaceutically acceptable salts thereof are disclosed, together with processes for their preparation. The compounds of formulae (I) are MPO inhibitors and are thereby particularly useful in the treatment or prophylaxis of neuroinflammatory disorders.
表征谱图
-
氢谱1HNMR
-
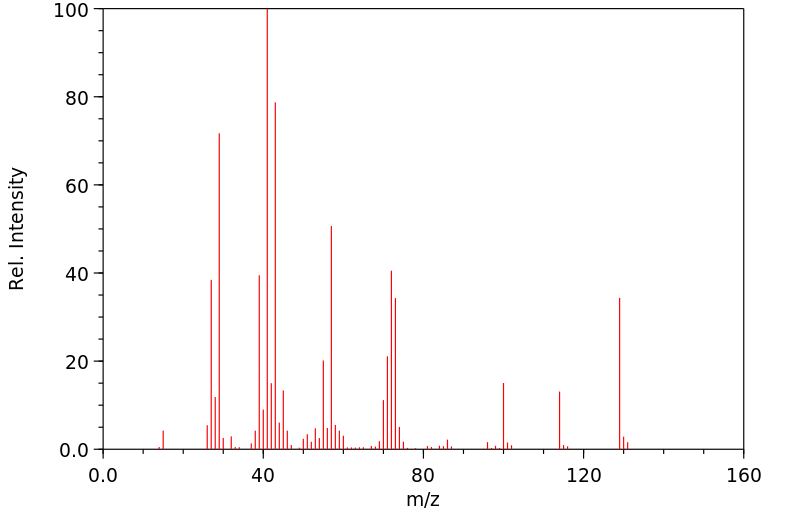
质谱MS
-
碳谱13CNMR
-
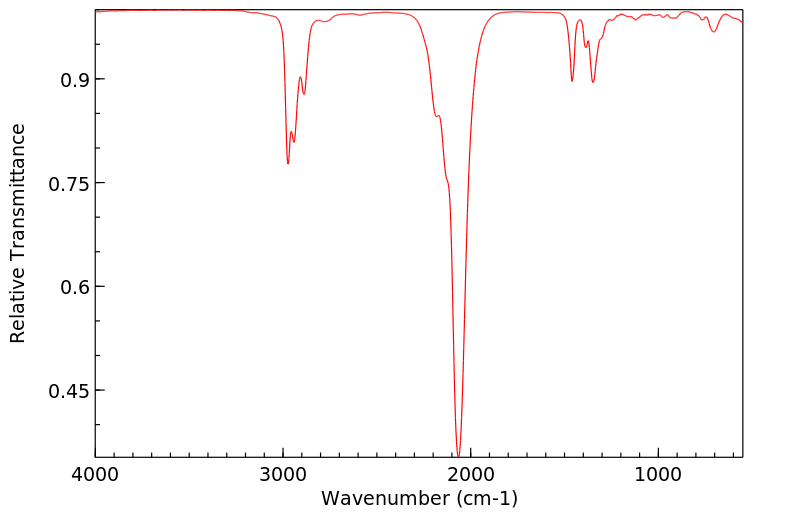
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-2-异硫氰基-1-环戊羧酸乙酯
顺-2-异硫氰基-1-环己烷羧酸乙酯
羰基异氰酸酯异硫氰酸酯
羰基二异硫氰酸酯
硫代异氰酸环戊酯
甲代烯丙基异硫氰酸酯
环己烷羰基异硫氰酸酯
环己基异硫氰酸脂
环丙基甲基异硫氰酸酯
环丁烷羰基异硫氰酸酯
异硫氰酸甲酯
异硫氰酸甲氧基甲酯
异硫氰酸甲基环己酯
异硫氰酸环辛酯
异硫氰酸环庚脂
异硫氰酸环十二酯
异硫氰酸环丙酯
异硫氰酸烯丙酯
异硫氰酸溴代乙酯
异硫氰酸氯代乙酯
异硫氰酸氨基甲酰
异硫氰酸异戊酯
异硫氰酸异丙酯
异硫氰酸异丁酯
异硫氰酸己酯
异硫氰酸叔辛酯
异硫氰酸十六酯
异硫氰酸十一烷酯
异硫氰酸仲丁酯
异硫氰酸乙酰酯
异硫氰酸乙酯
异硫氰酸乙烯
异硫氰酸3-丁烯酯
异硫氰酸2-甲氧基乙酯
异硫氰酸1-金刚烷酯
异硫氰酰甲酸甲酯
异硫氰酰甲酸乙酯
异硫氰基环丁烷
异硫代氰酰基乙醛二甲基乙缩醛
异氰酸丙酯
己烷,1-[(2-异硫氰基乙基)硫代]-
己烯,1-异硫氰基-
天然芥菜籽油
叔戊基异硫氰酸酯
叔丁基异硫氰酸酯
双(1-异硫氰基乙基)醚
十八烷基异氰酸酯
二(异硫氰酰甲基)醚
丙脒,N-丁基-2-氯-
三芥子酸甘油酯