[(3-甲基丁氧基)甲基]苯 | 122-73-6
中文名称
[(3-甲基丁氧基)甲基]苯
中文别名
苄基异戊基醚
英文名称
benzyl isoamyl ether
英文别名
((isopentyloxy)methyl)benzene;3-methylbutoxymethylbenzene
CAS
122-73-6
化学式
C12H18O
mdl
MFCD00048371
分子量
178.274
InChiKey
RXXCIBALSKQCAE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:236 °C
-
密度:0.91
-
闪点:110°C(lit.)
-
LogP:3.725 (est)
-
保留指数:1297;1309
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:13
-
可旋转键数:5
-
环数:1.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:9.2
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险品标志:Xn
-
危险类别码:R22
-
RTECS号:KM9620000
-
海关编码:2909309090
-
储存条件:盛满后应储存在阴凉、避光且通风的地方。
SDS
Benzyl Isoamyl Ether Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: Benzyl Isoamyl Ether
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Skin corrosion/irritation Category 3
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
None
Pictograms or hazard symbols
Signal word Warning
Causes mild skin irritation
Hazard statements
Precautionary statements:
If skin irritation occurs: Get medical advice/attention.
[Response]
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: Benzyl Isoamyl Ether
Percent: >98.0%(GC)
CAS Number: 122-73-6
Benzyl Isopentyl Ether
Synonyms:
Chemical Formula: C12H18O
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Get medical advice/attention if you feel unwell. Rinse mouth.
Ingestion:
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, carbon dioxide.
media:
Unsuitable extinguishing Water (It may scatter and spread fire.)
media:
Benzyl Isoamyl Ether
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Wash hands and face thoroughly after
handling.
Use a ventilation, local exhaust if vapour or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Form: Clear
Colorless - Almost colorless
Colour:
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
236°C
Boiling point/range:
Flash point: 110°C
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
0.91
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents] No data available
Benzyl Isoamyl Ether
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: skn-rbt 500 mg/24H MLD
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
KM9620000
RTECS Number:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
Benzyl Isoamyl Ether
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: Benzyl Isoamyl Ether
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Skin corrosion/irritation Category 3
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
None
Pictograms or hazard symbols
Signal word Warning
Causes mild skin irritation
Hazard statements
Precautionary statements:
If skin irritation occurs: Get medical advice/attention.
[Response]
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: Benzyl Isoamyl Ether
Percent: >98.0%(GC)
CAS Number: 122-73-6
Benzyl Isopentyl Ether
Synonyms:
Chemical Formula: C12H18O
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Get medical advice/attention if you feel unwell. Rinse mouth.
Ingestion:
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, carbon dioxide.
media:
Unsuitable extinguishing Water (It may scatter and spread fire.)
media:
Benzyl Isoamyl Ether
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Wash hands and face thoroughly after
handling.
Use a ventilation, local exhaust if vapour or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Form: Clear
Colorless - Almost colorless
Colour:
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
236°C
Boiling point/range:
Flash point: 110°C
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
0.91
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents] No data available
Benzyl Isoamyl Ether
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: skn-rbt 500 mg/24H MLD
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
KM9620000
RTECS Number:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
Benzyl Isoamyl Ether
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— (((3-methylbut-3-en-1-yl)oxy)methyl)benzene 58558-53-5 C12H16O 176.258 [双(3-甲基丁氧基)甲基]苯 benzaldehyde di(neopentyl) acetal 94231-95-5 C17H28O2 264.408 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 苯甲酸异戊酯 isoamyl benzoate 94-46-2 C12H16O2 192.258 —— [Dideuterio(3-methylbutoxy)methyl]benzene 948587-77-7 C12H18O 180.258
反应信息
-
作为反应物:描述:参考文献:名称:Masui, Masaichiro; Hara, Seijiro; Ueshima, Takahiro, Chemical and pharmaceutical bulletin, 1983, vol. 31, # 11, p. 4209 - 4212摘要:DOI:
-
作为产物:参考文献:名称:通过钛环丁烷的质子分解进行位点特异性烯烃氢甲基化摘要:甲基在生物活性分子中普遍存在。因此,将这种烷基片段引入多官能结构的新策略引起了人们的极大兴趣。考虑到这一目标,报告了一种烯烃马尔可夫尼科夫氢甲基化的直接方法。该方法利用了Cp 2 Ti(μ-Cl)(μ-CH 2 )AlMe 2 (Tebbe试剂)中的钛亚甲基与未活化的烯烃之间的简并复分解反应。所得环丁烷钛的原位质子解作用以化学、区域和位点选择性方式实现氢甲基化。该方法的广泛实用性在一系列含有侧醇、醚、酰胺、氨基甲酸酯和碱性胺的单取代和二取代烯烃中得到了证明。DOI:10.1002/anie.202103278
文献信息
-
[EN] HETEROARYL COMPOUNDS AND THEIR USE AS THERAPEUTIC DRUGS<br/>[FR] COMPOSÉS HÉTÉROARYLE ET LEUR UTILISATION COMME MÉDICAMENTS THÉRAPEUTIQUES申请人:DONG-A SOCIO HOLDINGS CO LTD公开号:WO2017039331A1公开(公告)日:2017-03-09The present invention provides heterocyclic compounds, the stereoisomer thereof, the enantiomer thereof, or the pharmaceutically acceptable salt, which are capable of modulating the activity of Mer receptor tyrosine kinase (MERTK). This invention also provides pharmaceutical compositions thereof, methods to prepare the said compounds, and the use of such compounds as a medicament. The present invention is directed to MERTK inhibitory compounds with marked potency, thereby having an outstanding potential for a pharmaceutical intervention of cancer and any other diseases related to MERTK dysregulation.
-
Process For Producing Dipeptides or Dipeptide Derivatives申请人:Hashimoto Shin-ichi公开号:US20080213827A1公开(公告)日:2008-09-04The present invention provides a process for producing a dipeptide or a dipeptide derivative by using a protein having the activity to form the dipeptide or dipeptide derivative from one or more kinds of amino acids or amino acid derivatives, or a culture of cells having the ability to produce the protein or a treated matter of the culture as a enzyme source, which comprise; allowing the enzyme source, one or more kinds of amino acids or amino acid derivatives and ATP to be present in an aqueous medium; allowing the dipeptide or dipeptide derivative to form and accumulate in the medium; and recovering the dipeptide or dipeptide derivative from the medium.
-
Tandem Ring-Closing Metathesis/Transfer Hydrogenation: Practical Chemoselective Hydrogenation of Alkenes作者:Timothy Connolly、Zhongyu Wang、Michael A. Walker、Ivar M. McDonald、Kevin M. PeeseDOI:10.1021/ol5019739日期:2014.9.5chemoselective transfer hydrogenation of alkenes using ruthenium metathesis catalysts is presented. Of great practicality, the transfer hydrogenation reagents can be added directly to a metathesis reaction and effect hydrogenation of the product alkene in a single pot at ambient temperature without the need to seal the vessel to prevent hydrogen gas escape. The reduction is applicable to a range of alkenes and
-
Reductive Etherification via Anion-Binding Catalysis作者:Chenfei Zhao、Christopher A. Sojdak、Wazo Myint、Daniel SeidelDOI:10.1021/jacs.7b05832日期:2017.8.2Reductive condensations of alcohols with aldehydes/ketones to generate ethers are catalyzed by a readily accessible thiourea organocatalyst that operates in combination with HCl. 1,1,3,3-tetramethyldisiloxane serves as a convenient reducing reagent. This strategy is applicable to challenging substrate combinations and exhibits functional group tolerance. Competing reductive homocoupling of the carbonyl
-
Efficient and Convenient Heterogeneous Palladium-Catalyzed Regioselective Deuteration at the Benzylic Position作者:Takanori Kurita、Kazuyuki Hattori、Saori Seki、Takuto Mizumoto、Fumiyo Aoki、Yuki Yamada、Kanoko Ikawa、Tomohiro Maegawa、Yasunari Monguchi、Hironao SajikiDOI:10.1002/chem.200701147日期:2008.1.7hydrogen-deuterium (H-D) exchange reaction on the benzylic site proceeded in D2O in the presence of a small amount of H2 gas. The use of the Pd/C-ethylenediamine complex [Pd/C(en)] as a catalyst instead of Pd/C led to the efficient deuterium incorporation into the benzylic site of O-benzyl protective groups without hydrogenolysis. These H-D exchange reactions provide a post synthetic and D(2)-gas-free deuterium-labeling
表征谱图
-
氢谱1HNMR
-
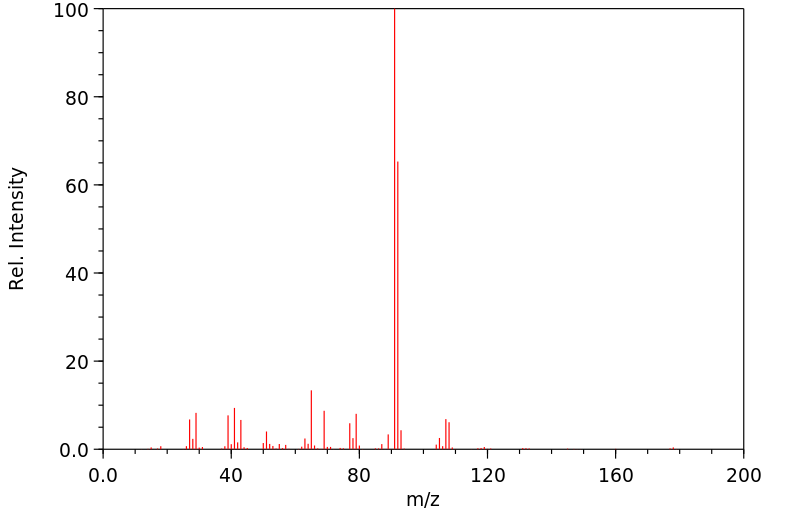
质谱MS
-
碳谱13CNMR
-
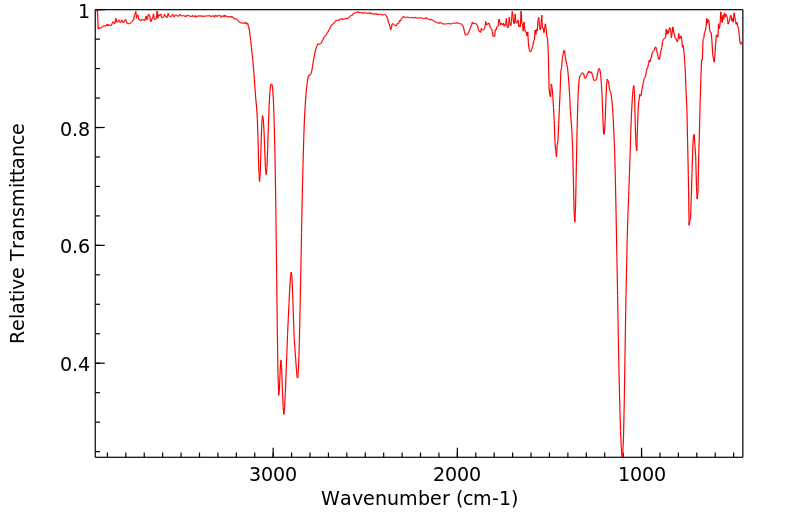
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫