1-异硫代氰酸庚酯 | 4426-83-9
中文名称
1-异硫代氰酸庚酯
中文别名
1-异硫氰酸庚酯;异硫氰酸庚酯
英文名称
1-heptyl isothiocyanate
英文别名
heptyl isothiocyanate;Heptylisothiocyanat;1-isothiocyanatoheptane
CAS
4426-83-9
化学式
C8H15NS
mdl
MFCD00041138
分子量
157.28
InChiKey
LIPUQNPCPLDDBO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:235 °C
-
密度:0.91
-
闪点:106 °C
-
LogP:4.191 (est)
-
保留指数:1253
-
稳定性/保质期:
常温常压下稳定,无色液体。
计算性质
-
辛醇/水分配系数(LogP):4.5
-
重原子数:10
-
可旋转键数:6
-
环数:0.0
-
sp3杂化的碳原子比例:0.875
-
拓扑面积:44.4
-
氢给体数:0
-
氢受体数:2
安全信息
-
TSCA:Yes
-
危险等级:6.1
-
危险品标志:Xn
-
危险类别码:R36/37/38
-
危险品运输编号:2810
-
海关编码:2930909090
-
包装等级:II
-
危险类别:6.1
-
安全说明:S26,S36/37/39
-
危险性防范说明:P260,P303+P361+P353,P305+P351+P338,P301+P330+P331,P405,P501
-
危险性描述:H302,H312,H331,H314
-
储存条件:请将药品存放在避光、通风干燥的地方,并密封保存。
SDS
| Name: | Heptyl Isothiocyanate Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 4426-83-9 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 4426-83-9 | Heptane, 1-Isothiocyanato- | ca 100 | 224-610-3 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.Moisture sensitive.The toxicological properties of this material have not been fully investigated.Lachrymator (substance which increases the flow of tears).
Potential Health Effects
Eye:
Causes eye irritation. Lachrymator (substance which increases the flow of tears). May cause chemical conjunctivitis.
Skin:
Causes skin irritation.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated.
Inhalation:
Causes respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. Can produce delayed pulmonary edema.
Chronic:
Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid. Do NOT allow victim to rub eyes or keep eyes closed.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Use water spray to keep fire-exposed containers cool. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas. Containers may explode when heated.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Do NOT get water inside containers. Cool containers with flooding quantities of water until well after fire is out. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Remove all sources of ignition. Provide ventilation. Do not get water inside containers.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. Do not allow contact with water. Keep from contact with moist air and steam.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Store protected from moisture.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 4426-83-9: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: colorless
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 235 deg C @ 760.00mm Hg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: 106 deg C ( 222.80 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: negligible in water
Specific Gravity/Density: .9100g/cm3
Molecular Formula: C8H15NS
Molecular Weight: 157.28
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, moisture, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Moisture, strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, oxides of nitrogen, oxides of sulfur, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 4426-83-9 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Heptane, 1-Isothiocyanato- - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: Toxic liquid, organic, n.o.s.*
Hazard Class: 6.1
UN Number: 2810
Packing Group: III
IMO
Shipping Name: Toxic liquid, organic, n.o.s.
Hazard Class: 6.1
UN Number: 2810
Packing Group: III
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 37/39 Wear suitable gloves and eye/face
protection.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 4426-83-9: No information available.
Canada
CAS# 4426-83-9 is listed on Canada's DSL List.
CAS# 4426-83-9 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 4426-83-9 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:参考文献:名称:Ponzio, Gazzetta Chimica Italiana, 1896, vol. 26 I, p. 326摘要:DOI:
-
作为产物:描述:参考文献:名称:脂肪族异硫氰酸酯类似物作为抗生素的合成及其构效关系摘要:异硫氰酸盐(ITC)是植物中发现的芥子油苷的多种分解产物之一,并且具有针对各种病原体的生物活性。在这项工作中,制备了脂族异硫氰酸酯,并测试了其对植物病原性真菌和细菌的抗菌活性,以了解其结构与活性之间的关系。结果表明施加空间抑制该长链衍生物关于对信托公司的毒性水稻纹枯病菌由于位阻和八个脂族的ITC的顺序为乙基>的 Ñ丙基>甲基> ñ -己基> Ñ辛基> ñ -丁基> 正庚基> n-戊基。由于ITC的疏水性通过增加烷基链长度而增强,因此ITC对胡萝卜欧文氏菌的抗菌活性中等,随着疏水性的增加而增强,其顺序为正辛基> 正戊基> 正庚基> 正己基> n丙基> 正丁基>甲基>乙基。目前的研究表明,一些化合物表现出令人信服的抗菌活性,可以作为控制茄螺和胡萝卜螺的传统合成杀菌剂的可接受替代品。DOI:10.1007/s00044-012-0323-4
文献信息
-
Synthesis and properties of some formylferrocene thiosemicarbazones作者:D. M. Wiles、T. SuprunchukDOI:10.1139/v68-310日期:1968.6.1
Twelve new substituted thiosemicarbazones have been prepared from formylferrocene and 2- and 4-monosubstituted and 4,4-disubstituted thiosemicarbazides. The infrared spectra of these compounds are discussed in some detail. Copper complexes of three of them were synthesized and their structures established. They were found to be strongly paramagnetic. The electronic absorption spectra of the thiosemicarbazones and the copper complexes are reported.
-
Substituted Spiro Compounds and Their Use for Producing Pain-Relief Medicaments申请人:Frank Robert公开号:US20080269271A1公开(公告)日:2008-10-30The present invention relates to substituted spiro compounds, to processes for preparing them, to medicaments comprising these compounds and to the use of these compounds for producing medicaments.本发明涉及替代螺环化合物,涉及制备这些化合物的方法,涉及含有这些化合物的药物以及利用这些化合物生产药物的用途。
-
Antihemolytic and Anticonvulsant Activities of 1-(2,4-Dichloro/2,4,5-Trichlorophenoxyacetyl)-4-alkyl/arylthiosemicarbazides and Their Inhibition of NAD-Dependent Oxidations and Monoamine Oxidase作者:Basheer Ali、Rajendra Kumar、Surendra S. Parmar、Chandradhar Dwivedi、Raymond D. HarbisonDOI:10.1002/jps.2600640815日期:1975.8oxidation of succinate was not affected. These compounds inhibited the activity of monamine oxidase in rat brain homogenate, and the degree of inhibition ranged from 26.5 to 89.2 percent at a final concentration of 0.03mM, with kynuramine as the substrate. Almost all thiosemicarbazides possessed anticonvulsant activity; protection against pentylenetetrazol-induced convulsions in mice ranged from 10 to 70合成了几种1-(2,4-二氯和2,4,5-三氯苯氧基乙酰基)-4-烷基/芳硫代氨基脲,并通过其敏锐的熔点和元素分析对其进行了表征。所有取代的硫代氨基脲可保护狗红细胞的体外低渗溶血。这些硫代氨基脲选择性抑制丙酮酸和α-酮戊二酸的烟酰胺腺嘌呤二核苷酸(NAD)依赖性氧化,而琥珀酸的NAD依赖性氧化则不受影响。这些化合物抑制大鼠脑匀浆中单胺氧化酶的活性,在终浓度为0.03mM的情况下,以犬尿嘧啶为底物,其抑制程度范围为26.5%至89.2%。几乎所有的硫代氨基脲都具有抗惊厥活性。剂量为100 mg / kg ip时,对戊四氮诱发的小鼠惊厥的保护作用范围为10%至70%。这些结果提供了这些取代的硫代氨基脲的膜稳定性能与其对NAD依赖的氧化的选择性抑制和对单胺氧化酶的抑制能力的相似性的证据。另一方面,这些取代的硫代氨基脲所具有的抗惊厥活性与它们的体外抗溶血和酶抑制特性无关。
-
Synthesis and Antihypertensive Activity of N-(Alkyl/alkenyl/aryl)-N'-heterocyclic Ureas and Thioureas作者:Opa Vajragupta、Aungkana Pathomsakul、Chutima Matayatsuk、Lek Ruangreangyingyod、Yuvadee Wongkrajang、William O. FoyeDOI:10.1021/js930295x日期:1996.3significant antihypertensive activity (p < 0.05). 1-n-Propyl-3-[2'-(6-methoxy)quinolyl]urea (9), showing 29.1% reduction in systolic blood pressure, was the most active compound in the series. Two other compounds producing a fall in systolic blood pressure of the same magnitude were 1-allyl-3-[2'-(6-methyl)quinolyl]thiourea (4) and 1-n-propyl-3-[(2'-pyridyl)methyl]urea (17). Compound 17 with rapid
-
Biocidal isothiourea compounds申请人:Stauffer Chemical Company公开号:US04233318A1公开(公告)日:1980-11-11Novel biocidal S-aminoalkyl isothiourea compounds have the general structural formula ##STR1## wherein X is halogen; R is a straight or branched chain alkyl radical containing 5 to 20 carbon atoms; R.sub.1 is selected from the group consisting of alkyl containing 1 to 20 carbon atoms, alkenyl containing 2 to 6 carbon atoms, benzyl, halo substituted benzyl, .alpha.-methyl benzyl, furfuryl and pyridyl methyl; R.sub.2 is hydrogen or alkyl containing 1 to 10 carbon atoms; R.sub.3 and R.sub.4 are independently hydrogen or alkyl containing 1 to 4 carbon atoms; and R.sub.5 is divalent alkylene containing 2 to 4 carbon atoms.
表征谱图
-
氢谱1HNMR
-
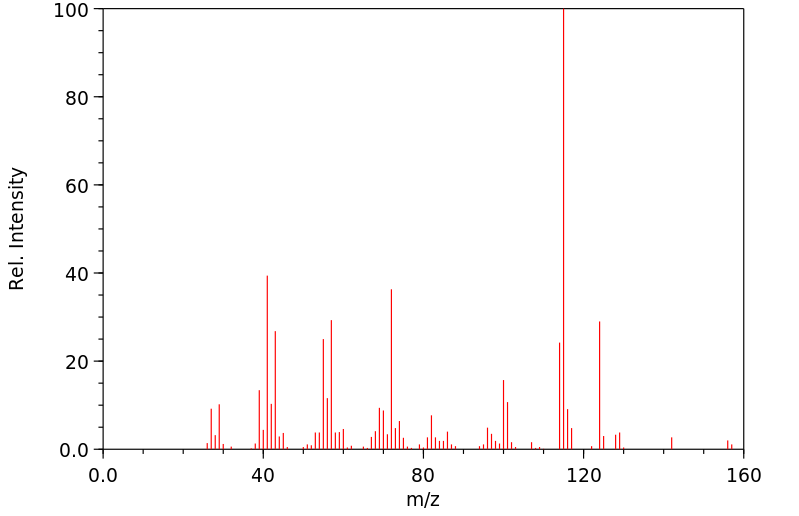
质谱MS
-
碳谱13CNMR
-
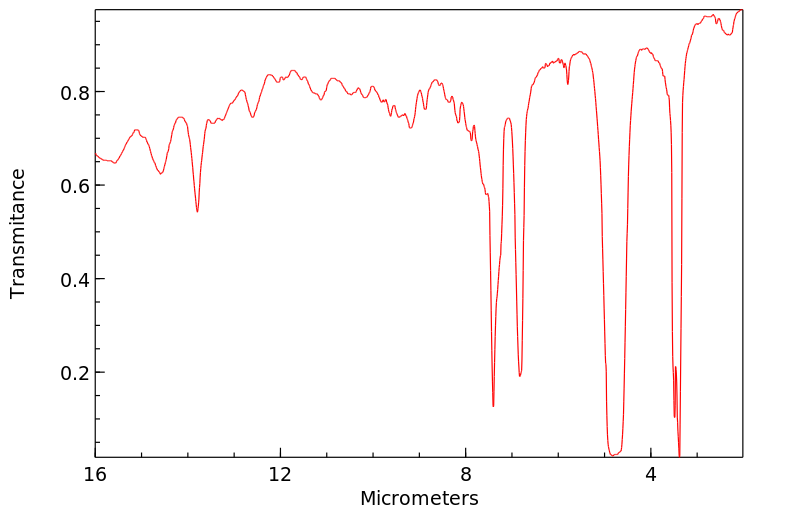
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-2-异硫氰基-1-环戊羧酸乙酯
顺-2-异硫氰基-1-环己烷羧酸乙酯
羰基异氰酸酯异硫氰酸酯
羰基二异硫氰酸酯
硫代异氰酸环戊酯
甲代烯丙基异硫氰酸酯
环己烷羰基异硫氰酸酯
环己基异硫氰酸脂
环丙基甲基异硫氰酸酯
环丁烷羰基异硫氰酸酯
异硫氰酸甲酯
异硫氰酸甲氧基甲酯
异硫氰酸甲基环己酯
异硫氰酸环辛酯
异硫氰酸环庚脂
异硫氰酸环十二酯
异硫氰酸环丙酯
异硫氰酸烯丙酯
异硫氰酸溴代乙酯
异硫氰酸氯代乙酯
异硫氰酸氨基甲酰
异硫氰酸异戊酯
异硫氰酸异丙酯
异硫氰酸异丁酯
异硫氰酸己酯
异硫氰酸叔辛酯
异硫氰酸十六酯
异硫氰酸十一烷酯
异硫氰酸仲丁酯
异硫氰酸乙酰酯
异硫氰酸乙酯
异硫氰酸乙烯
异硫氰酸3-丁烯酯
异硫氰酸2-甲氧基乙酯
异硫氰酸1-金刚烷酯
异硫氰酰甲酸甲酯
异硫氰酰甲酸乙酯
异硫氰基环丁烷
异硫代氰酰基乙醛二甲基乙缩醛
异氰酸丙酯
己烷,1-[(2-异硫氰基乙基)硫代]-
己烯,1-异硫氰基-
天然芥菜籽油
叔戊基异硫氰酸酯
叔丁基异硫氰酸酯
双(1-异硫氰基乙基)醚
十八烷基异氰酸酯
二(异硫氰酰甲基)醚
丙脒,N-丁基-2-氯-
三芥子酸甘油酯