2,3-戊二烯 | 591-96-8
中文名称
2,3-戊二烯
中文别名
對稱二甲基丙二烯
英文名称
penta-2,3-diene
英文别名
2,3-Pentadien;2,3-pentadiene;Penta-2,3-dien;1.3-Dimethyl-allen;Pentadien-(2.3);1,3-Dimethylallene
CAS
591-96-8
化学式
C5H8
mdl
——
分子量
68.1185
InChiKey
PODAMDNJNMAKAZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-125.65°C
-
沸点:48.2°C
-
密度:0.6900
-
保留指数:544;531;556
计算性质
-
辛醇/水分配系数(LogP):1.3
-
重原子数:5
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Synthesis of Some Alkylidenecyclopropanes from Allenes1摘要:DOI:10.1021/jo01341a029
-
作为产物:描述:alkaline earth salt of/the/ methylsulfuric acid 在 aluminum oxide 、 cis-decahydronaphthalene 、 sodium 作用下, 生成 2,3-戊二烯参考文献:名称:从烯烃合成丙二烯的两步法摘要:按两步顺序进行,其中第一步涉及将二溴卡宾加成到烯烃中,而第二步涉及所得的取代的1,1-二溴环丙烷与镁或钠的反应,得到了丙二烯。总体结构变化涉及在原始双键的两个之间插入一个碳原子,因此代表了一种将碳链长度增加一个原子的新颖方法。DOI:10.1016/0040-4020(58)88025-4
文献信息
-
Reactions of cumulated and conjugated dienes with sulfur trioxide作者:Ruud M. Schonk、Bert H. Bakker、Hans CerfontainDOI:10.1002/recl.19931120301日期:——The reactions of a series of cumulated (1a-5a) and conjugated alkadienes (8a-13a) with sulfur trioxide have been studied in the temperature range of −60 to 25°C using dichloromethane as solvent and 1.5 equiv of dioxane as reactivity moderator. Reaction of tetramethylallene (1a) at low temperature yielded the β-sultone 1b, which at 0°C was slowly converted into 2,4-dimethyl-1,3-pentadiene-3-sulfonic在-60至25°C的温度范围内,使用二氯甲烷作为溶剂,使用1.5当量的二恶烷作为反应调节剂,研究了一系列累积的(1a-5a)和共轭链二烯(8a-13a)与三氧化硫的反应。四甲基丙烯烯(1a)在低温下的反应产生β-磺内酯1b,其在0℃下缓慢转化为2,4-二甲基-1,3-戊二烯-3-磺酸(1f)。在另外的SO 3存在下,该共轭二烯磺酸在0℃下迅速转化为不饱和δ-磺内酯1g。1,2-环壬二烯(5a)与SO 3的反应作为通常观察到的不饱和δ-磺内酯g的形成的一个例外,因为在低温下形成2-环壬烯-2,1-磺内酯(5b)和相应的羰基硫酸盐(5d)的1:1混合物。将这些产物在0℃下均转化为相同的链二烯磺酸,即。6。当使用过量的SO 3时,6被迅速转化为3-环壬烯-1,2-磺内酯-3-磺酸(7)。
-
The reactions of benzyne with allenes
-
Etude expérimentale et théorique de l'addition de l'acide hypochloreux sur les hydrocarbures alléniques作者:J.-P. Bianchini、M. CocordanoDOI:10.1016/s0040-4020(01)92917-5日期:——The addition of hypochlorous acid to allenic hydrocarbons leads, in all cases, to the fixation of the Cl atom on the central carbon and to the fixation of the OH group on the more substituted carbon. The (α-chloroketones which might be produced by the inverse fixation are not obtained. The utilisation of Hückel's method explains these results theoretically and satisfactorily.
-
The silyl-cupration and stannyl-cupration of allenes作者:Ian Fleming、Michael Rowley、Purificacíon Cuadrado、Ana M. González-Nogal、Francisco J. PulidoDOI:10.1016/0040-4020(89)80069-9日期:1989.1The stoichiometric silyl-cupration of allene 7, followed directly by treating the intermediate cuprate with a proton, with a range of carbon electrophiles, and with chlorine gives the vinylsilanes 8–13. Alternatively, when iodine is the electrophile, the product is the vinyl iodide 16. This can then be metallated and treated with a proton or a range of
-
Enantioselective photochemistry through Lewis acid–catalyzed triplet energy transfer作者:Travis R. Blum、Zachary D. Miller、Desiree M. Bates、Ilia A. Guzei、Tehshik P. YoonDOI:10.1126/science.aai8228日期:2016.12.16spectroscopic data rule out substrate activation by means of photoinduced electron transfer and instead support a mechanism in which Lewis acid coordination dramatically lowers the triplet energy of the chalcone substrate. We expect that this approach will enable chemists to more broadly apply their detailed understanding of chiral Lewis acid catalysis to stereocontrol in reactions involving electronically excited
表征谱图
-
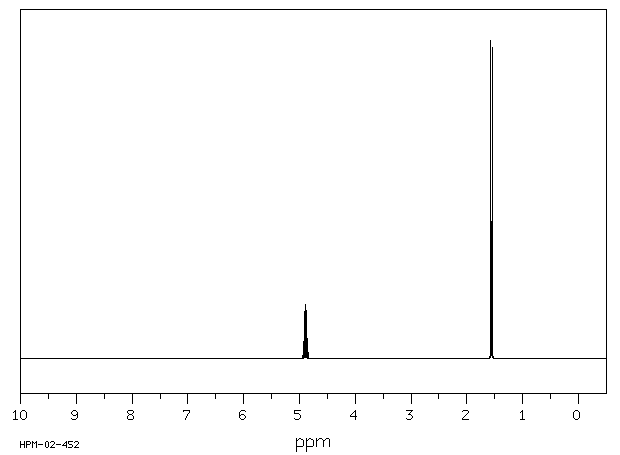
氢谱1HNMR
-
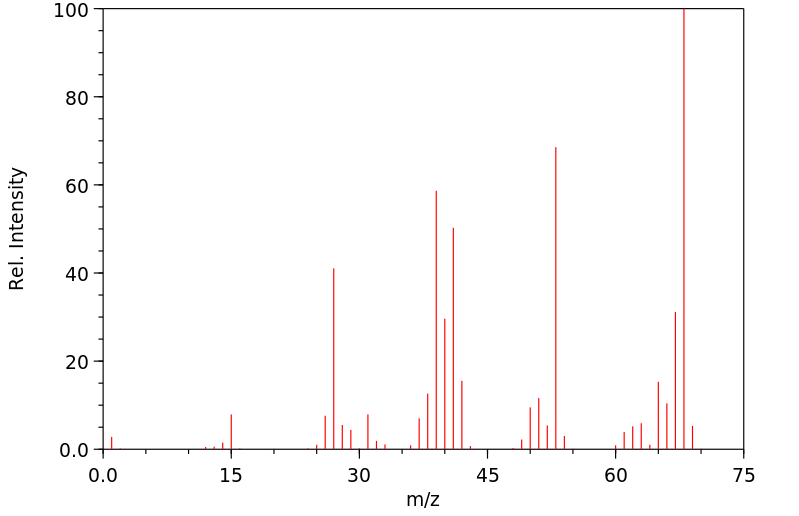
质谱MS
-
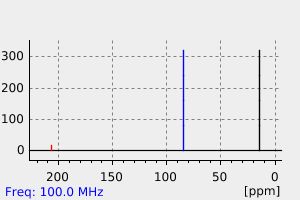
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
辛-3,4-二烯
癸-3,4-二烯
癸-2,3-二烯
癸-1,2-二烯
炔丙基自由基
庚-1,2-二烯
己-1,2,4,5-四烯
壬-4,5-二烯
十二碳-2,3-二烯
十二碳-1,2-二烯
十三碳-4,5-二烯
十一碳-1,3,4-三烯
十一碳-1,2-二烯
丙二烯-d4
丙二烯
C=C=C-H自由基
7-甲基辛-3,4-二烯
7,7-二甲基辛-3,4-二烯
6,6-二甲基-庚-2,3-二烯
5-甲基-1,2-己二烯
5,6-十一碳二烯
4-甲基戊-1,2-二烯
4-甲基-1,2,5-己三烯
3,4-庚二烯
3,4-壬二烯
2-甲基-4,5-壬二烯
2,3-辛二烯
2,3-戊二烯
2,3-壬二烯
2,2-二甲基-1,3,5-己三烯
2,2,6,6-四甲基庚-3,4-二烯
1-叔丁基丙二烯
1-二乙基膦基-戊二烯-(2,3)
1,2-辛二烯
1,2-戊二烯
1,2-己二烯
1,2-壬二烯
1,2-十三碳二烯
1,2-丁二烯
1,2,5-己三烯
1,2,4-戊三烯
1,2,3-丁三烯
1-Diaethylphosphino-hexadien-(2.3)
1-pentyl-3-pentylallene
tert-butyl-3-deuterioallene
penta-2,3-diene
2-methylundeca-3,4-diene
2-Methyl-nonatrien-(5,6,8)
(4,4-dimethylpenta-1,2-dien-1-yl)silver
2,2-dimethyl-3,4-heptadiene