6-(二丁基氨基)-1,3,5-三唑-2,4-二硫醇 | 29529-99-5
中文名称
6-(二丁基氨基)-1,3,5-三唑-2,4-二硫醇
中文别名
油酸二丁铵;6-(二丁氨基)-1,3,5-三嗪-2,4-二硫醇;6-(二丁基氨基)-1,3,5-三嗪-2,4(1H,3H)-二硫酮
英文名称
2-di-n-butylamino-1,3,5-triazine-4,6-dithiol
英文别名
6-(dibutylamino)-1,3,5-triazine-2,4-dithiol;6-dibutylamino-S-triazine-2,4-dithiol;1,3,5-Triazine-2,4(1H,3H)-dithione, 6-(dibutylamino)-;6-(dibutylamino)-1H-1,3,5-triazine-2,4-dithione
CAS
29529-99-5
化学式
C11H20N4S2
mdl
——
分子量
272.439
InChiKey
IXDGHAZCSMVIFX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:143 °C
-
沸点:343.7±25.0 °C(Predicted)
-
密度:1.22±0.1 g/cm3(Predicted)
-
溶解度:可溶于DMSO(少许)、甲醇(少许)
-
LogP:2.2 at 40℃ and pH2.1
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,也未有已知危险反应。
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:17
-
可旋转键数:7
-
环数:1.0
-
sp3杂化的碳原子比例:0.73
-
拓扑面积:104
-
氢给体数:2
-
氢受体数:2
安全信息
-
海关编码:2933699090
-
包装等级:III
-
危险类别:9
-
危险性防范说明:P280,P301+P312+P330,P305+P351+P338+P310,P273
-
危险品运输编号:3077
-
危险性描述:H302,H318,H412
-
储存条件:保持贮藏器密封,并将其存放在阴凉、干燥处。确保工作间有良好的通风或排气装置。
SDS
6-(Dibutylamino)-1,3,5-triazine-2,4-dithiol Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 6-(Dibutylamino)-1,3,5-triazine-2,4-dithiol
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 6-(Dibutylamino)-1,3,5-triazine-2,4-dithiol
Percent: >98.0%(LC)(N)
CAS Number: 29529-99-5
Synonyms: 2-(Dibutylamino)-4,6-dimercapto-1,3,5-triazine
Chemical Formula: C11H20N4S2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
6-(Dibutylamino)-1,3,5-triazine-2,4-dithiol
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: Crystal- Powder
Colour: White - Very pale yellow
Odour: No data available
pH: No data available
Melting point/freezing point:143°C
No data available
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
No data available
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents]
Pyridine
Soluble:
6-(Dibutylamino)-1,3,5-triazine-2,4-dithiol
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx), Sulfur oxides
products:
Section 11. TOXICOLOGICAL INFORMATION
No data available
Acute Toxicity:
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
No data available
Reproductive toxicity:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
6-(Dibutylamino)-1,3,5-triazine-2,4-dithiol
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 6-(Dibutylamino)-1,3,5-triazine-2,4-dithiol
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 6-(Dibutylamino)-1,3,5-triazine-2,4-dithiol
Percent: >98.0%(LC)(N)
CAS Number: 29529-99-5
Synonyms: 2-(Dibutylamino)-4,6-dimercapto-1,3,5-triazine
Chemical Formula: C11H20N4S2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
6-(Dibutylamino)-1,3,5-triazine-2,4-dithiol
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: Crystal- Powder
Colour: White - Very pale yellow
Odour: No data available
pH: No data available
Melting point/freezing point:143°C
No data available
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
No data available
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents]
Pyridine
Soluble:
6-(Dibutylamino)-1,3,5-triazine-2,4-dithiol
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx), Sulfur oxides
products:
Section 11. TOXICOLOGICAL INFORMATION
No data available
Acute Toxicity:
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
No data available
Reproductive toxicity:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
6-(Dibutylamino)-1,3,5-triazine-2,4-dithiol
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:尿苷(5')二氢二磷酰(1)-alpha-D-葡萄糖 、 6-(二丁基氨基)-1,3,5-三唑-2,4-二硫醇 在 glycosyltransferases YdhE bacillus licheniformi 、 magnesium chloride 作用下, 以 二甲基亚砜 为溶剂, 反应 3.0h, 以3.6 mg的产率得到6-dibutyl-amino 2-S-β-D-glucopyranosyl 1,3,5 triazine参考文献:名称:两种三官能的卢卢尔糖基转移酶作为天然产物糖多样化的生物催化剂。摘要:两种混杂的地衣芽孢杆菌糖基转移酶YdhE和YojK表现出20种不同类别的59种结构不同的天然和非天然产物的突出的立体特异性但非区域特异性的糖基化活性。两种酶都在各种化合物的三个亲核基团(OH,NH 2,SH)上转移了各种糖,从而生成O-,N-和S-糖苷。这些酶还显示了一锅转糖基化的催化可逆性潜力,因此在药物发现中赋予了糖多样化天然产物生物合成的经济有效应用。DOI:10.1021/acs.orglett.9b03040
-
作为产物:描述:三聚氯氰 、 二正丁胺 在 sodium carbonate 、 sodium hydrogen sulfide 作用下, 以 乙醇 、 水 为溶剂, 反应 3.0h, 以87.28%的产率得到6-(二丁基氨基)-1,3,5-三唑-2,4-二硫醇参考文献:名称:一锅法合成医用胶塞/活塞用2-二正丁胺基-1,3,5-三嗪-4,6-二硫醇的方法摘要:本发明提供一种一锅法合成医用胶塞/活塞用三嗪二硫醇类交联剂2‑二正丁胺基‑1,3,5‑三嗪‑4,6‑二硫醇的方法。具体包括以下步骤:在反应瓶中,加入醇类溶剂和三聚氯氰,再缓慢加入二正丁胺;滴加完毕,加入合适的缚酸剂调节pH值;撤掉冷冻盐水,通过恒压加料装置缓慢加入预先备好的硫氢化钠水溶液进行巯基化反应;用酸调节pH值至白色沉淀完全析出;冷却至室温,抽滤,滤饼水洗至中性,真空干燥,得到目标产物,溶剂蒸馏回收后套用。本发明采用一锅法工艺,克服了传统方法合成工艺繁琐、使用有毒有害溶剂的缺点,操作简单方便,合成的产品色泽浅,收率高,熔点≥140℃,纯度≥99.0%,满足药用胶塞/活塞领域对高性能制品的需求。公开号:CN111377876A
文献信息
-
Terpyridine platinum(ii) complexes containing triazine di- or tri-thiolate bridges: structures, luminescence, electrochemistry, and aggregation作者:Hua-Xin Zhang、Masako Kato、Yoichi Sasaki、Tadashi Ohba、Hiroto Ito、Atsushi Kobayashi、Ho-Chol Chang、Kohei UosakiDOI:10.1039/c2dt30997a日期:——Dinuclear complexes [Pt(trpy)}2(L)](PF6)2 (trpy = 2,2′:6′,2′′-terpyridine, L = 2-octylthio-1,3,5-triazine-4,6-dithiolate ion (1), L = 2-octadecylthio-1,3,5-triazine-4,6-dithiolate ion (2), L = 2-di-n-butylamino-1,3,5-triazine-4,6-dithiolate ion (3)) and a trinuclear complex [Pt(trpy)}3(L)](PF6)3 (L = 1,3,5-triazine-2,4,6-trithiolate ion (4)) have been synthesized and characterized. The single crystal X-ray analysis revealed that the two Pt(trpy)}2+ fragments in 1 and 3 adopt a syn-configuration. The Pt⋯Pt distances are around 4.3 Å, suggesting no intramolecular Pt⋯Pt interactions. Complexes 1–4 in acetonitrile show broad absorption bands at around 470 nm, assigned to mainly the ligand-to-ligand charge transfer (1LLCT) from triazine thiolates to trpy based on the comparison to the related complexes and the density functional theory (DFT) calculations. The red luminescence of 1–4 in acetonitrile is attributable to emission predominantly from 3LLCT. Cyclic voltammograms of 1–3 exhibit four redox couples from −2.0 V to 0 V vs. Ag/AgCl. The two consecutive processes at around −0.70 V are assigned to the sequential reduction of two trpy ligands. This assignment was further supported by the observation of the anion radical of trpy in spectroelectrochemical experiments. The splitting of the redox potentials of two trpy ligands evidences the moderate electronic coupling interactions mediated by the triazine dithiolate bridges. Complex 2 formed a transparent red gel in CH3CN, whereas 4 produced a gel-like solid in the mixtures of CH3CN and other solvents. The interactions dominating the aggregative behaviours have been discussed based on the results of electronic absorption and emission spectroscopy.合成并表征了双核配合物 [Pt(trpy)}2(L)](PF6)2(trpy = 2,2′:6′,2′′-三吡啶,L = 2-辛基硫-1,3,5-三嗪-4,6-二硫醋酸根离子 (1),L = 2-十八烷基硫-1,3,5-三嗪-4,6-二硫醋酸根离子 (2),L = 2-二正丁基氨基-1,3,5-三嗪-4,6-二硫醋酸根离子 (3))和三核配合物 [Pt(trpy)}3(L)](PF6)3(L = 1,3,5-三嗪-2,4,6-三硫醋酸根离子 (4)。单晶X射线分析显示,配合物1和3中的两个 Pt(trpy)}2+片段呈现出syn构型。Pt⋯Pt距离约为4.3 Å,表明不存在分子内的Pt⋯Pt相互作用。配合物1-4在乙腈中呈现出约470 nm的宽吸收带,主要归因于三嗪二硫醋酸根到trpy之间的配体间电荷转移(1LLCT),这基于与相关配合物及密度泛函理论(DFT)计算的比较。配合物1-4在乙腈中的红光发射主要源于3LLCT。配合物1-3的循环伏安图显示,从−2.0 V到0 V与Ag/AgCl比较,存在四对氧化还原对。在约−0.70 V处的两个连续过程被归因于两个trpy配体的顺序还原。这一归因在光谱电化学实验中通过观察trpy的阴离子自由基进一步得到了支持。两个trpy配体的氧化还原电势的分裂证明了由三嗪二硫醋酸盐桥介导的适度电子耦合相互作用。配合物2在CH3CN中形成透明红色凝胶,而4则在CH3CN与其他溶剂的混合物中产生类似凝胶的固体。根据电子吸收和发射光谱的结果,讨论了主导聚集行为的相互作用。
-
Thiolate-based One-dimensional Flexible Pb–MOFs Exhibiting a Large Sorption Hysteresis Phenomenon作者:Yoshinobu Kamakura、Ryo Hamano、Yuiga Nakamura、Kunihisa Sugimoto、Hirofumi Yoshikawa、Daisuke TanakaDOI:10.1246/cl.210054日期:2021.5.5A novel thiolate-based Pb-metal-organic framework, [Pb2(Hdtd)4·2DMSO]n (KGF-2·DMSO, H2dtd = 6-dibutylamino-1,3,5-triazine-2,4-dithol and DMSO = dimethyl sulfoxide) was prepared. The crystal structure of KGF-2·DMSO consists of a one-dimensional chain composed of Pb ions and surrounding ligands. Following removal of the guest DMSO molecules, KGF-2 was obtained as a crystalline powder, which exhibited
-
Thermoplastic resin composition and elastomer composition申请人:——公开号:US20040106732A1公开(公告)日:2004-06-03Provided are a thermoplastic resin composition which is excellent in oil resistance, heat resistance, weatherability, impact resistance, transparency, and moldability, and which can be produced economically, an elastomer composition with low hardness and high tensile strength which is excellent in oil resistance and compression set, and a molded object produced by molding the thermoplastic resin composition or elastomer composition. The composition is produced by compounding a thermoplastic resin or an elastomer with a block copolymer having at least one methacrylic ester polymer block and at least one acrylic ester polymer block. Also provided are a process for producing a methacrylic ester-acrylic ester-methacrylic ester block copolymer which requires hardly any purification, which is excellent in heat resistance and weatherability, and in which the molecular weight and the molecular-weight distribution are controlled, and a methacrylic ester-acrylic ester-methacrylic ester block copolymer produced by the process.
-
Polymer separation membrane申请人:——公开号:US20030110947A1公开(公告)日:2003-06-19A separation membrane comprising a polyether polymer having a weight-average molecular weight of 10 4 to 10 7 and obtained by polymerizing an oxirane compound of the formula: 1 wherein R is a hydrogen atom; an alkyl group, an alkyl group having an halogen atom, or —CH 2 O(CH 2 CH 2 O) k —R′ (R′ is a group selected from an alkyl group, an alkenyl group, a cycloalkyl group, an aryl group and an aralkyl group; k is from 0 to 12.); or an ethylenically unsaturated group, a reactive silicon-containing group or a methylepoxy-containing group, is excellent in mechanical strength and flexibility.
-
RESIN COMPOSITION AND SEMICONDUCTOR DEVICE BOARD申请人:Osaku Yumi公开号:US20140087136A1公开(公告)日:2014-03-27A resin composition which contains a binder resin (A), radiation-sensitive compound (B), silane-modified resin (C), and antioxidant (D), wherein a content of the silane-modified resin (C) is 0.1 to 150 parts by weight with respect to 100 parts by weight of the binder resin (A), a content of the antioxidant (D) is 0.1 to 10 parts by weight with respect to 100 parts by weight of the binder resin (A), and an amount of warping is 14 μm or less when using the resin composition to form a thickness 2 to 3 μm resin film and baking the formed resin film at 230° C. is provided.
表征谱图
-
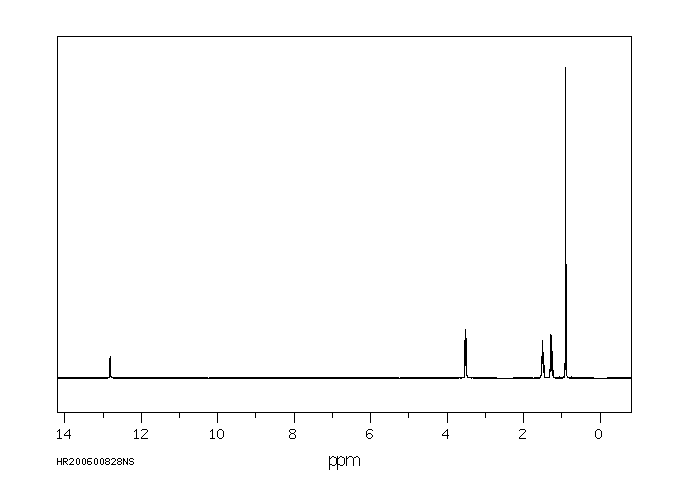
氢谱1HNMR
-
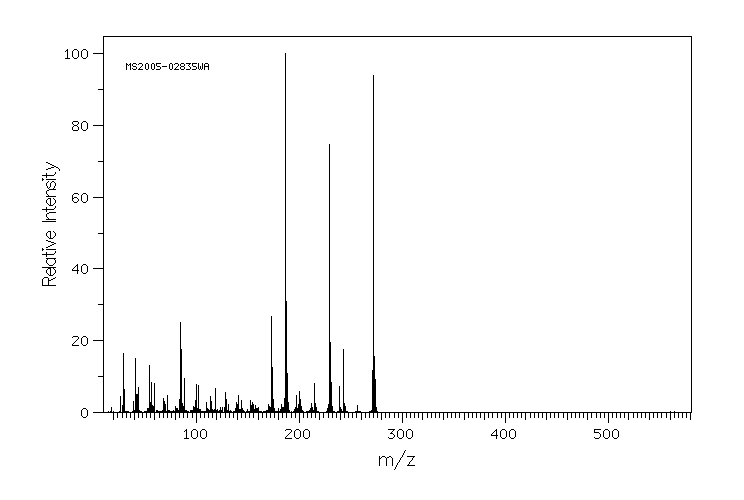
质谱MS
-
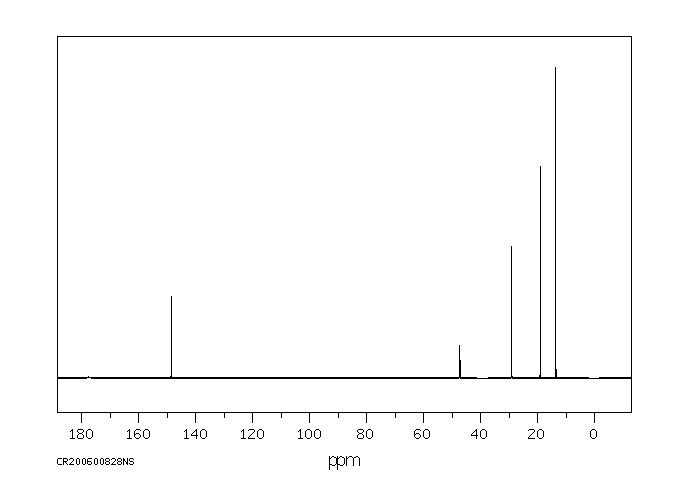
碳谱13CNMR
-
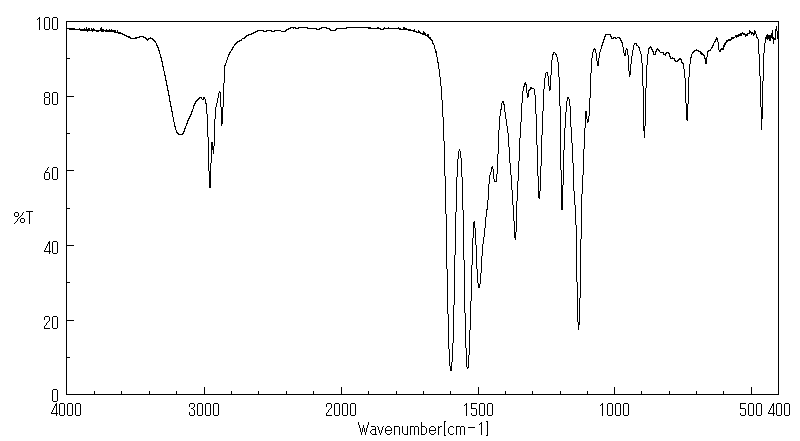
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷