5-氯噻吩-2-甲醛 | 7283-96-7
中文名称
5-氯噻吩-2-甲醛
中文别名
5-氯-2-噻吩甲醛;2-氯-5-噻吩甲醛;5-氯-2-噻酚缩醛;2-羟基-3,4-二氟苯胺2-羟基-3,4-二氟苯胺
英文名称
5-Chloro-2-thiophenecarboxaldehyde
英文别名
5-chlorothiophene-2-carbaldehyde;5-chlorothiophene-2-carboxaldehyde
CAS
7283-96-7
化学式
C5H3ClOS
mdl
MFCD00047090
分子量
146.597
InChiKey
VWYFITBWBRVBSW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:99 °C/21 mmHg (lit.)
-
密度:1.376 g/mL at 25 °C (lit.)
-
闪点:208 °F
-
溶解度:可溶于氯仿(少许)、甲醇(少许)
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解。
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:8
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:45.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
TSCA:Yes
-
危险品标志:Xn
-
危险类别码:R20/21/22
-
WGK Germany:3
-
海关编码:2934999090
-
安全说明:S24,S26,S36,S36/37/39
-
危险标志:GHS07
-
危险性描述:H302,H312,H332
-
危险性防范说明:P280
-
储存条件:密封储存于阴凉干燥处。
SDS
| Name: | 5-Chloro-2-thiophenecarboxaldehyde 97% Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 7283-96-7 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 7283-96-7 | 2-Thiophenecarboxaldehyde, 5-chloro- | 97 | 230-708-7 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation. The toxicological properties of this material have not been fully investigated.
Skin:
May cause skin irritation. The toxicological properties of this material have not been fully investigated.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Never give anything by mouth to an unconscious person. Get medical aid immediately.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Keep container closed when not in use. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use process enclosure, local exhaust ventilation, or other engineering controls to control airborne levels.
Exposure Limits CAS# 7283-96-7: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: clear almost colorless
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 89 deg C @ 9.00mm Hg
Freezing/Melting Point: 0 deg C
Autoignition Temperature: Not available.
Flash Point: 94 deg C ( 201.20 deg F)
Explosion Limits, lower: N/A
Explosion Limits, upper: N/A
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C5H3ClOS
Molecular Weight: 146.60
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Chlorine, carbon monoxide, oxides of sulfur, irritating and toxic fumes and gases, carbon dioxide, chloride fumes.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 7283-96-7 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2-Thiophenecarboxaldehyde, 5-chloro- - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 7283-96-7: No information available.
Canada
CAS# 7283-96-7 is listed on Canada's NDSL List.
CAS# 7283-96-7 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 7283-96-7 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
应用
5-氯噻吩-2-甲醛是一种油状液体,具有类似苦杏仁的气味,在医药及中间体方面有广泛应用。例如,作为抗肿瘤用药(替尼泊苷)、广谱高效驱肠寄生虫药(噻嘧啶)和保肝药(替尼酮)。
合成方法
在250 mL反应瓶中加入1 mol/L 5-氯噻吩溶液、1.2 mol/L DMF,并投入1 g 催化剂,搅拌下升温至50~55℃。均匀通入光气40 g/h,2.5 h后取样进行中控分析,采用气谱面积归一法测定噻吩与噻吩甲醛的百分比含量,直至噻吩含量低于1%时停止通光气,并改为通氮气以去除多余的光气。持续2小时后,降温至30℃以下,加入100 mL冷水搅拌0.5 h,随后进行水蒸气蒸馏并分离出有机相。通过精馏塔减压蒸馏,最终得到噻吩甲醛产品,其纯度超过99%,收率大于90%。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-氯-5-甲基噻吩 2-chloro-5-methylthiophene 17249-82-0 C5H5ClS 132.614 2-噻吩甲醛 thiophene-2-carbaldehyde 98-03-3 C5H4OS 112.152 2-氯-5-氯甲基噻吩 2-Chloro-5-(chloromethyl)thiophene 23784-96-5 C5H4Cl2S 167.059 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-氯-5-甲基噻吩 2-chloro-5-methylthiophene 17249-82-0 C5H5ClS 132.614 5-氯-2-噻吩羧胺 5-chlorothiophene-2-carboxamide 22353-82-8 C5H4ClNOS 161.612 2-氯噻吩-5-甲酸 5-Chlorothiophene-2-carboxylic acid 24065-33-6 C5H3ClO2S 162.597 4,5-二氯-2-噻吩羧醛 4,5-dichlor-2-thiophenecarboxaldehyde 67482-49-9 C5H2Cl2OS 181.042 5-氯-2-噻吩甲醇 (5-chlorothiophen-2-yl)methanol 74168-69-7 C5H5ClOS 148.613 2-(溴甲基)-5-氯噻吩 2-(bromomethyl)-5-chlorothiophene 59311-22-7 C5H4BrClS 211.51 5-氯噻吩-2-甲酸甲酯 methyl 5-chloro-2-thiophenecarboxylate 35475-03-7 C6H5ClO2S 176.624 5-氯-2-噻吩甲腈 5-chlorothiophene-2-carbonitrile 50478-16-5 C5H2ClNS 143.597 2-氯-5-氯甲基噻吩 2-Chloro-5-(chloromethyl)thiophene 23784-96-5 C5H4Cl2S 167.059
反应信息
-
作为反应物:描述:参考文献:名称:Fournari,P.; Chane,J.P., Bulletin de la Societe Chimique de France, 1963, p. 479 - 484摘要:DOI:
-
作为产物:描述:参考文献:名称:肼-O2氧化还原对作为有机催化氧化的平台:苯并[c] cinnoline催化的烷基卤化物氧化为醛类摘要:描述了一种有机催化氧化平台,该平台利用了肼快速自氧化为二氮烯的能力。在涉及亲核攻击,质变和水解的新型机理范式中,显示出可商购的苯并[ c ]肉桂啉催化烷基卤化物氧化为醛。仅使用不定形的氧气和水就容易发生水解和再氧化事件。显示了对可行底物范围的调查,以及对这种催化方式有深入了解的机理和计算研究。DOI:10.1002/anie.201807134
文献信息
-
Diversity Oriented Clicking (DOC): Divergent Synthesis of SuFExable Pharmacophores from 2‐Substituted‐Alkynyl‐1‐Sulfonyl Fluoride (SASF) Hubs作者:Christopher J. Smedley、Gencheng Li、Andrew S. Barrow、Timothy L. Gialelis、Marie‐Claire Giel、Alessandra Ottonello、Yunfei Cheng、Seiya Kitamura、Dennis W. Wolan、K. Barry Sharpless、John E. MosesDOI:10.1002/anie.202003219日期:2020.7.20Diversity Oriented Clicking (DOC) is a unified click‐approach for the modular synthesis of lead‐like structures through application of the wide family of click transformations. DOC evolved from the concept of achieving “diversity with ease” , by combining classic C−C π‐bond click chemistry with recent developments in connective SuFEx‐technologies. We showcase 2‐Substituted‐Alkynyl‐1‐Sulfonyl Fluorides面向多样性的点击 (DOC) 是一种统一的点击方法,用于通过应用广泛的点击转换家族来模块化合成类先导结构。DOC 从实现“轻松实现多样性”的概念演变而来,将经典的 C−C π 键点击化学与连接性 SuFEx 技术的最新发展相结合。我们展示了 2-取代的- A炔基-1-磺酰基F氟化物(SASFs)作为一类新的连接枢纽,与多种点击环加成过程相结合。通过具有一系列偶极子和环状二烯的 SASF 的选择性 DOC,我们以最少的合成步骤报告了 173 种独特功能分子的多样化点击库。SuFExable 库包含 10 个离散的杂环核心结构,这些核心结构源自 1,3- 和 1,5- 偶极子;而与环状二烯的反应会产生几种三维双环 Diels-Alder 加合物。通过对 96 孔板中的磺酰氟侧基进行 SuFEx 点击衍生化,可以通过后期修饰将文库增加到 278 种离散化合物——证明了 DOC 方法在快速合成不同功能结构方面的多功能性。
-
3-Aminocyclopentanecarboxamides as Modulators of Chemokine Receptors申请人:Xue Chu-Biao公开号:US20070149532A1公开(公告)日:2007-06-28The present invention is directed to compounds of Formula I: I which are modulators of chemokine receptors. The compounds of the invention, and compositions thereof, are useful in the treatment of diseases related to chemokine receptor expression and/or activity.
-
Application of the Intramolecular Diels–Alder Vinylarenе (IMDAV) Approach for the Synthesis of Thieno[2,3-f]isoindoles作者:Mykola D. Obushak、Fedor I. Zubkov、Maryana A. Nadirova、Yevhen-Oleh V. Laba、Vladimir P. Zaytsev、Julya S. Sokolova、Kuzma M. Pokazeev、Victoria A. Anokhina、Victor N. Khrustalev、Yuriy I. Horak、Roman Z. Lytvyn、Miłosz Siczek、Vasyl Kinzhybalo、Yan V. Zubavichus、Maxim L. KuznetsovDOI:10.1055/s-0039-1690833日期:2020.83-(Thien-2-yl)- and 3-(thien-3-yl)allylamines, readily accessible from the corresponding thienyl aldehydes, can interact with a broad range of anhydrides and α,β-unsaturated acids chlorides (maleic, сitraconic, and phenyl maleic anhydrides, сrotonyl and сinnamyl chlorides, etc.) leading to the formation of a thieno[2,3-f]isoindole core. Usually, the reaction sequence involves three successive steps:很容易从相应的噻吩基醛中获得的3-(噻吩-2-基)-和3-(噻吩-3-基)烯丙胺可以与各种酸酐和α,β-不饱和酰氯(马来酸,顺丁烯二酸)相互作用,以及苯基马来酸酐,叔丁酰和肉桂酰氯等),导致形成噻吩并[2,3- f ]异吲哚核。通常,反应顺序包括三个连续的步骤:初始烯丙胺的氮原子的酰化,分子内Diels-Alder乙烯基芳烃(IMDAV)反应以及Diels-Alder加合物中二氢噻吩环的最终芳构化。彻底研究了该方法的范围和局限性。借助X射线分析表明,关键步骤IMDAV反应通过exo进行。-过渡态,引起目标杂环的单一非对映异构体的排他形成。在马来酸酐的情况下,该方法允许获得官能取代的噻吩并[2,3- f ]异吲哚羧酸,其对于进一步转化和随后的生物筛选是潜在有用的底物。
-
Effect of organic solvents on solvatochromic, fluorescence, and electrochemical properties of synthesized thiazolylcoumarin derivatives作者:Ali Bahadur、Shahid Iqbal、Rabail Ujan、Pervaiz Ali Channar、Murefah Mana AL‐Anazy、Aamer Saeed、Qaiser Mahmood、Muhammad Shoaib、Mazloom Shah、Ifzan Arshad、Ghulam Shabir、Muhammad Saifullah、Guocong Liu、Muhammad Abdul QayyumDOI:10.1002/bio.4044日期:——of thiazolylcoumarin derivatives with bioactive scaffolds was confirmed through nuclear magnetic resonance spectroscopy. A solvatochromic study of synthesized thiazolylcoumarin derivatives was carried out using ultraviolet–visible methods for dimethylformamide (DMF), ethyl acetate, and ethanol solvents. The redox behaviour of as-synthesized thiazolylcoumarin derivatives (5a–5k) was examined in dimethyl
-
Highly Efficient Monophosphine-Based Catalyst for the Palladium-Catalyzed Suzuki−Miyaura Reaction of Heteroaryl Halides and Heteroaryl Boronic Acids and Esters作者:Kelvin Billingsley、Stephen L. BuchwaldDOI:10.1021/ja068577p日期:2007.3.1active and efficient catalyst system derived from a palladium precatalyst and monophosphine ligands 1 or 2 for the Suzuki-Miyaura cross-coupling reaction of heteroaryl boronic acids and esters has been developed. This method allows for the preparation of a wide variety of heterobiaryls in good to excellent yields and displays a high level of activity for the coupling of heteroaryl chlorides as well as
表征谱图
-
氢谱1HNMR
-
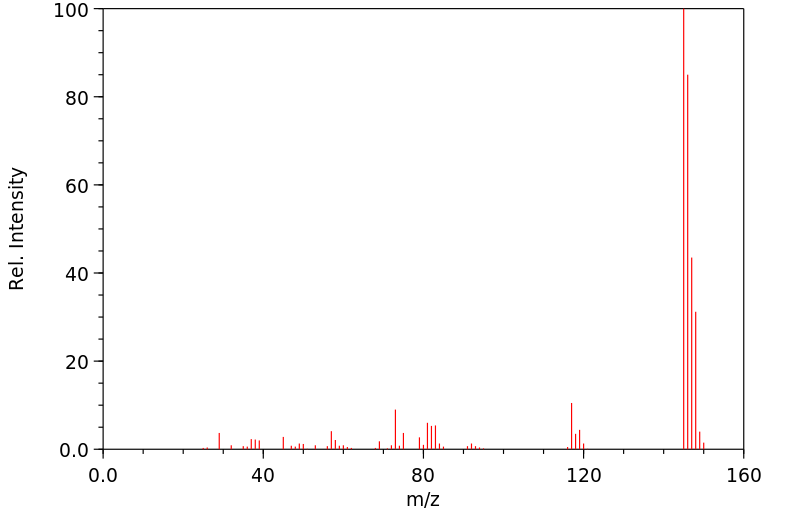
质谱MS
-
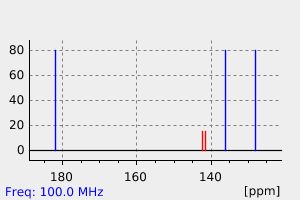
碳谱13CNMR
-
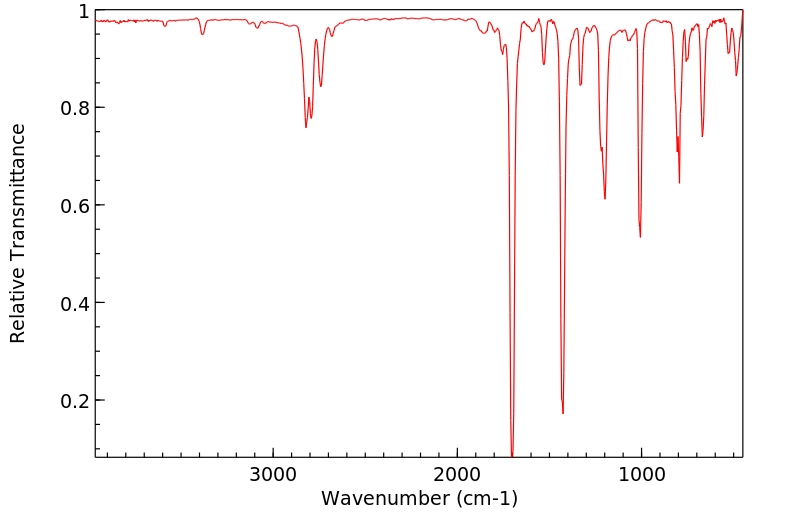
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
阿罗洛尔
阿替卡因
阿克兰酯
锡烷,(5-己基-2-噻吩基)三甲基-
邻氨基噻吩(2盐酸)
辛基5-(1,3-二氧戊环-2-基)-2-噻吩羧酸酯
辛基4,6-二溴噻吩并[3,4-b]噻吩-2-羧酸酯
辛基2-甲基异巴豆酸酯
血管紧张素IIAT2受体激动剂
葡聚糖凝胶LH-20
苯螨噻
苯并[c]噻吩-1-羧酸,5-溴-4,5,6,7-四氢-3-(甲硫基)-4-羰基-,乙基酯
苯并[b]噻吩-2-胺
苯并[b]噻吩-2-胺
苯基-[5-(4,4,5,5-四甲基-[1,3,2]二氧杂硼烷-2-基)-噻吩-2-基亚甲基]-胺
苯基-(5-氯噻吩-2-基)甲醇
苯乙酸,-α--[(1-羰基-2-丙烯-1-基)氨基]-
苯乙酰胺,3,5-二氨基-a-羟基-2,4,6-三碘-
苯乙脒,2,6-二氯-a-羟基-
腈氨噻唑
聚(3-丁基噻吩-2,5-二基),REGIOREGULAR
硝呋肼
硅烷,(3-己基-2,5-噻吩二基)二[三甲基-
硅噻菌胺
盐酸阿罗洛尔
盐酸阿罗洛尔
盐酸多佐胺
甲酮,[5-(1-环己烯-1-基)-4-(2-噻嗯基)-1H-吡咯-3-基]-2-噻嗯基-
甲基5-甲酰基-4-甲基-2-噻吩羧酸酯
甲基5-乙氧基-3-羟基-2-噻吩羧酸酯
甲基5-乙基-3-肼基-2-噻吩羧酸酯
甲基5-(氯甲酰基)-2-噻吩羧酸酯
甲基5-(氯乙酰基)-2-噻吩羧酸酯
甲基5-(氨基甲基)噻吩-2-羧酸酯
甲基5-(4-甲氧基苯基)-2-噻吩羧酸酯
甲基5-(4-甲基苯基)-2-噻吩羧酸酯
甲基5-(1,3-二氧戊环-2-基)-2-噻吩羧酸酯
甲基4-硝基-2-噻吩羧酸酯
甲基4-氰基-5-(4,6-二氨基吡啶-2-基)偶氮-3-甲基噻吩-2-羧酸酯
甲基4-氨基-5-(甲硫基)-2-噻吩羧酸酯
甲基4-{[(2E)-2-(4-氰基苯亚甲基)肼基]磺酰}噻吩-3-羧酸酯
甲基4-(氯甲酰基)-3-噻吩羧酸酯
甲基4-(氨基磺酰基氨基)-3-噻吩羧酸酯
甲基3-甲酰氨基-4-甲基-2-噻吩羧酸酯
甲基3-氨基-5-异丙基-2-噻吩羧酸酯
甲基3-氨基-5-(4-溴苯基)-2-噻吩羧酸酯
甲基3-氨基-4-苯基-5-(三氟甲基)-2-噻吩羧酸酯
甲基3-氨基-4-氰基-5-甲基-2-噻吩羧酸酯
甲基3-氨基-4-丙基-2-噻吩羧酸酯
甲基3-[[(4-甲氧基苯基)亚甲基氨基]氨基磺酰基]噻吩-2-羧酸酯