2-嘧啶硫代乙酸 | 88768-45-0
中文名称
2-嘧啶硫代乙酸
中文别名
2-(羧基甲基硫代)嘧啶
英文名称
2-(carboxymethylthio)pyrimidine
英文别名
2-pyrimidylthiolylacetic acid;(2-pyrimidylthio)acetic acid;pyrimidine-2-thioacetic acid;2-pyrimidylthioacetic acid;(2-pyrimidinylthio)acetic acid;pyrimidin-2-ylmercapto-acetic acid;2-pyrimidin-2-ylsulfanylacetic acid
CAS
88768-45-0
化学式
C6H6N2O2S
mdl
MFCD00010287
分子量
170.192
InChiKey
NIEOYUNNKKAQKI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:204 °C (dec.) (lit.)
-
沸点:356.1±25.0 °C(Predicted)
-
密度:1.382 (estimate)
-
LogP:0.648 (est)
-
溶解度:>25.5 [ug/mL]
-
稳定性/保质期:
按规定使用和贮存的物质不会分解,并应避免与氧化物、碱接触。
计算性质
-
辛醇/水分配系数(LogP):0.6
-
重原子数:11
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.166
-
拓扑面积:88.4
-
氢给体数:1
-
氢受体数:5
安全信息
-
危险品标志:Xi
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2933599090
-
安全说明:S26,S37/39
-
危险标志:GHS07
-
危险性描述:H315,H319,H335
-
危险性防范说明:P261,P305 + P351 + P338
-
储存条件:请将药品存放在密闭、阴凉、干燥的地方。
SDS
| Name: | (2-Pyrimidylthio)Acetic Acid 98% Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 88768-45-0 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 88768-45-0 | (2-Pyrimidylthio)Acetic Acid | 98% | 289-445-1 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation. May cause chemical conjunctivitis.
Skin:
Causes skin irritation.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated.
Inhalation:
Causes respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. Can produce delayed pulmonary edema.
Chronic:
Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Minimize dust generation and accumulation. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing.
Keep container tightly closed. Avoid ingestion and inhalation. Use with adequate ventilation. Wash clothing before reuse.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 88768-45-0: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: off-white - yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 200 - 203 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: soluble in ethylacetate, acetone
Specific Gravity/Density:
Molecular Formula: C6H6N2O2S
Molecular Weight: 170.19
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, oxides of nitrogen, oxides of sulfur, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 88768-45-0 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
(2-Pyrimidylthio)Acetic Acid - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 88768-45-0: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 88768-45-0 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 88768-45-0 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:参考文献:名称:一些新的1,2,4-三唑席夫碱和1,2,4-三唑[3,4-b] [1,3,4]噻二嗪的合成,表征和抗菌活性摘要:背景:在本研究中,合成了14个新的1,2,4-三唑希夫碱和8个新的并入嘧啶环的1,2,4-三唑并[3,4-b] [1,3,4]噻二嗪,并评估其抗菌活性。 方法:在()存在下,由4-氨基-3-巯基-5-(2-硫甲基嘧啶基)-1,2,4-三唑与一系列芳醛缩合制备1,2,4-三唑席夫碱。 +)-酒石酸作为催化剂。另一方面,使4-氨基-3-巯基-5-(2-硫甲基嘧啶基)-1,2,4-三唑与取代的苯甲酰溴反应,得到相应的1,2,4-三唑并[3,4- b] [1,3,4]-噻二嗪。 结果:通过IR,NMR和质谱数据表征了1,2,4-三唑席夫碱和1,2,4-三唑并[3,4-b] [1,3,4]噻二嗪的结构。使用96孔微肉汤稀释法筛选所有合成的化合物的抗菌活性。 结论:具有对羟基苯基取代基的席夫碱和含4-氟苯环的三唑并噻二嗪在MIC 62.5μg/ ml时对铜绿假单胞菌具有中等的抗菌活性。DOI:10.2174/1570178614666171129160503
-
作为产物:描述:参考文献:名称:Synthesis and Biological Study of Some Novel 4-[5-(4,6-Disubstituted-2-thiomethylpyrimidyl)-4′-amino-1,2,4- triazol-3′-yl] thioacetyl-3-arylsydnones摘要:A series of 4-[5-(4,6-disubstituted-2-thiomethyl pyrimidyl)-4'-amino-1,2-4-triazol-3'-yl]thioacetyl-3-arylsydnones 7a-i were synthesized by the reaction of 5-(4,6-disubstituted-2-thiomethylpyrimidyl)-4-amino-3-mercapto-1,2-4-triazoles 3 with 3-aryl-4-bromoacetyl sydnones 6 in an ethanol medium. The newly synthesized compounds 7a-i were screened for their antibacterial activity against E. coli and Serratia marcesens and for antibacterial activity against Aspergillus niger and Pencillium. Most of the tested compounds showed significant antifungal activity particularly against Pencillium at 10-mu g/mL concentration comparable with that of the standard drug Flukanazole.DOI:10.1080/10426500601161049
文献信息
-
Synthetic chemical diversity: solid phase synthesis of libraries of C2 symmetric inhibitors of HIV protease containing diamino diol and diamino alcohol cores作者:Gary T. Wang、Sam Li、Norman Wideburg、Grant A. Krafft、Dale J. KempfDOI:10.1021/jm00016a001日期:1995.8structure--activity relationship (SAR) studies. To expand the scope of solid phase synthesis beyond the capability of the traditional method of solid phase synthesis for peptides, a strategy was developed for bi-directional solid phase synthesis starting with diamino alcohol or diamino diol core structures. The strategy relies on using bifunctional linkers to modify the core structures, simultaneously protecting为了高效率地产生用于药物筛选的大量结构多样的化合物,一直在追求非低聚有机化合物的固相合成。这些化合物被称为化学多样性文库或组合文库(当以组合方式进行合成时),可用于从头发现药物前导或方便的结构-活性关系(SAR)研究。为了将固相合成的范围扩展到传统的肽固相合成方法的能力之外,开发了一种从二氨基醇或二氨基二醇核心结构开始的双向固相合成策略。该策略依靠使用双功能链接程序来修改核心结构,同时保护核的羟基或二醇部分,并提供羧基以将修饰的核连接到固体载体上。然后将与固相支持物相连的修饰核的两个NH2基团脱保护,并与多种胺反应试剂(羧酸,磺酰氯,异氰酸酯,氯甲酸酯等)反应,以在两个方向上扩展分子。此策略已成功应用于包含已知的基于对称性的二氨基二醇和二氨基醇核心结构的HIV蛋白酶C2对称抑制剂文库的自动平行合成,因此可以方便地访问此系列中的大量类似物。使用此方法合成了300多种离散化合物的文库,以鉴定有效的(IC50
-
Antibiotic oxazolidinone derivatives申请人:Zeneca Ltd.公开号:US06365751B1公开(公告)日:2002-04-02The invention concerns a compound of the formula (I): wherein, for example: R1 is of the formula —NHC(═O)Rb wherein Rb is, for example, (1-4C)alkyl; R2 and R3 are hydrogen or fluoro; R2 and R3 are hydrogen or fluoro; D is O; R4 and R5 are hydrogen, (1-4C)alkyl or AR-oxymethyl; AR is phenyl or phenyl(1-4C)alkyl; R6 is hydrogen; >A—B— is of the formula >C═C(Ra)—, >CHCHRa—, or >C(OH)CHRa— (> represents two single bonds) wherein Ra is hydrogen or (1-4C)alkyl; and pharmaceutically-acceptable salts thereof; processes for their preparation; pharmaceutical compositions containing them and their use as antibacterial agents.本发明涉及如下公式(I)的化合物:其中,例如:R1是如下公式的形式—NHC(═O)Rb,其中Rb例如为(1-4C)烷基;R2和R3是氢或氟;D是氧;R4和R5是氢,(1-4C)烷基或AR-甲氧基甲基;AR是苯基或苯基(1-4C)烷基;R6是氢;>A—B—是如下公式的形式>C═C(Ra)—, >CHCHR_a—, 或 >C(OH)CHR_a—(>代表两个单键),其中Ra是氢或(1-4C)烷基;以及它们的药用可接受盐;它们的制备过程;含有它们的药物组合物以及它们作为抗菌剂的用途。
-
Synthesis and Structure−Activity Relationships of <i>N</i>-(1-Benzylpiperidin-4-yl)arylacetamide Analogues as Potent σ<sub>1</sub> Receptor Ligands作者:Yunsheng Huang、Philip S. Hammond、Li Wu、Robert H. MachDOI:10.1021/jm010384j日期:2001.12.1of the phenyl ring of the phenylacetamide moiety with a thiophene, naphthyl, or indole aromatic ring had no significant effect on the sigma1 receptor affinity. Replacement of the phenyl ring with an imidazole or pyridyl aromatic ring resulted in a >60-fold loss in affinity for sigma1 receptors and no significant binding affinity for sigma2 receptors. Substitution on the aromatic ring of the benzyl合成了一系列N-(1-苄基哌啶-4-基)芳基乙酰胺,并评估了它们与sigma1和sigma2受体的结合特性。与先前报道的N-(1-苄基哌啶-4-基)苯基乙酰胺的sigma1 / sigma2受体结合数据一致,下面报告的所有N-(1-苄基哌啶-4-基)芳基乙酰胺化合物均显示出对sigma1的亲和力高于sigma2受体。用噻吩,萘基或吲哚芳环取代苯乙酰胺部分的苯环对sigma1受体的亲和力没有明显影响。用咪唑或吡啶基芳环取代苯环会导致对sigma1受体的亲和力损失> 60倍,并且对sigma2受体的结合亲和力不明显。苄基芳香环上的取代显示出对sigma1受体的亲和力相似或略有降低。苯乙酰胺部分和苄基上的芳香环都被卤素取代,导致对sigma(1)受体的亲和力相似,并且对sigma2受体的亲和力大大提高。比较分子场分析表明,苯乙酰胺芳环中取代基的静电性质强烈影响与sigma1受体的结合。化合物1、
-
Compounds derived from aryl carbamates, preparation thereof and uses of same申请人:Maillet Magali公开号:US20050267126A1公开(公告)日:2005-12-01The invention concerns novel compounds, preparation and uses of same, particularly therapeutic. More particularly, the invention concerns compounds derived from arylcarbamates, preparation and uses of same, particularly in the field of human or animal health. The inventive compounds are preferably ligands of serotoninergic 5-HT4 receptors, and can therefore be used in the therapeutic or prophylactic treatment of any disorder involving a 5-HT4 receptor. The invention also relates to pharmaceutical compositions comprising such compounds, preparation and uses thereof, and treatment methods using said compounds.
-
Imidazonaphthyridines申请人:3M Innovative Properties Company公开号:US06194425B1公开(公告)日:2001-02-27Imidazonaphthyridine and tetrahydroimidazonaphthyridine compounds induce the biosynthesis of cytokines such as interferon and tumor necrosis factor. The compounds exhibit antiviral and antitumor properties. Methods of preparing the compounds and intermediates useful in the preparation of the compounds are also disclosed.
表征谱图
-
氢谱1HNMR
-
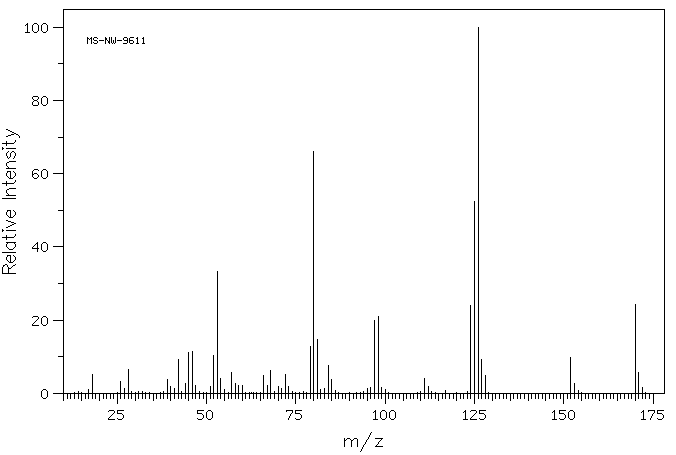
质谱MS
-
碳谱13CNMR
-
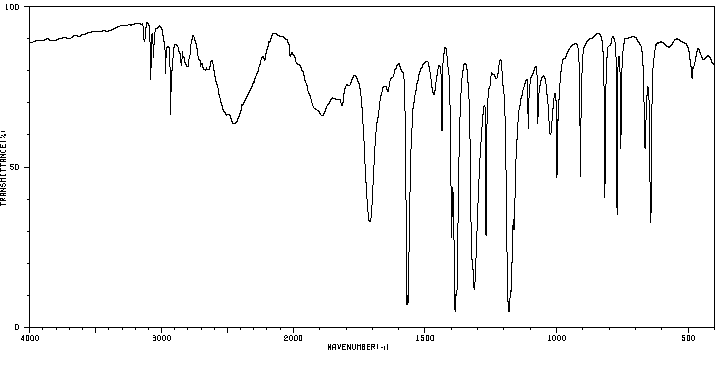
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Rp)-2-(叔丁硫基)-1-(二苯基膦基)二茂铁
(1E)-1-{4-[(4-氨基苯基)硫烷基]苯基}乙酮肟
颜料红88
颜料紫36
顺式-1,2-二(乙硫基)-1-丙烯
非班太尔-D6
雷西那得中间体
阿西替尼杂质J
阿西替尼杂质C
阿西替尼杂质4
阿西替尼杂质
阿西替尼
阿拉氟韦
阿扎毒素
阿嗪米特
阔草特
银(I)(6-氨基-2-(甲硫基)-5-亚硝基嘧啶-4-基)酰胺水合物
钾三氟[3-(苯基硫基)丙基]硼酸酯(1-)
邻甲苯基(对甲苯基)硫化物
避虫醇
连翘脂苷B
还原红 41
还原紫3
还原桃红R
达索尼兴
辛硫醚
辛-1,7-二炔-1-基(苯基)硫烷
西嗪草酮
萘,2-[(2,3-二甲基苯基)硫代]-
莫他哌那非
茴香硫醚
苯醌B
苯酰胺,N-(氨基亚氨基甲基)-4-[(2-甲基苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,N-(氨基亚氨基甲基)-4-[(2-氯苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,N-(氨基亚氨基甲基)-4-[(2,6-二氯苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,2-[(2-硝基苯基)硫代]-
苯酚,3-氯-4-[(4-硝基苯基)硫代]-
苯酚,3-(乙硫基)-
苯酚,3,5-二[(苯基硫代)甲基]-
苯胺,4-[5-溴-3-[4-(甲硫基)苯基]-2-噻嗯基]-
苯胺,3-氯-4-[(1-甲基-1H-咪唑-2-基)硫代]-
苯胺,2-[(2-吡啶基甲基)硫代]-
苯硫醚-D10
苯硫胍
苯硫基乙酸
苯硫代磺酸S-(三氯乙烯基)酯
苯甲醇,2,3,4,5,6-五氟-a-[(苯基硫代)甲基]-,(R)-
苯甲酸,3-[[2-[(二甲氨基)甲基]苯基]硫代]-,盐酸
苯甲胺,5-氟-2-((3-甲氧苯基)硫代)-N,N-二甲基-,盐酸
苯甲二硫酸,4-溴苯基酯