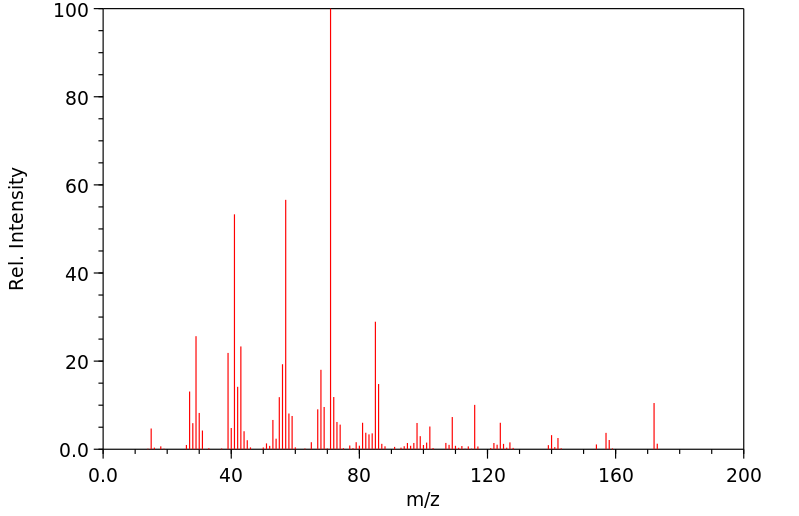
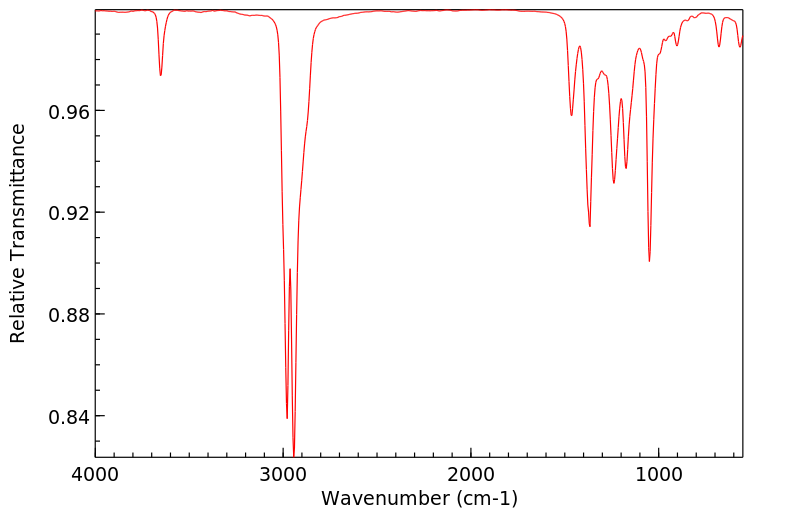
代谢
不同结构的硝基化合物在电子自旋共振(ESR)研究中的代谢,无论是在体外还是体内,通过气相色谱和气相色谱-质谱技术结合S波段ESR在人类角质形成细胞系HaCaT中进行了研究。除了已知的硝基自由基被还原为ESR静默的羟基胺作为初级产物外,我们的结果还表明形成了相应的二级胺。这些还原反应被巯基阻断剂N-乙基马来酰亚胺和强力的硫氧还蛋白还原酶(TR)抑制剂2-氯-2,4-硝基苯和2,6-二氯靛酚所抑制。竞争性TR抑制剂壬二酸和细胞色素P-450抑制剂美替拉酮没有效果。还原为羟基胺和二级胺的速率取决于硝基化合物的脂溶性。因此,可以假设硝基化合物必须进入细胞才能进行生物还原。被广泛讨论的细胞内硝基化合物还原物质抗坏血酸和谷胱甘肽无法形成二级胺。总之,我们的结果提示,二级胺是硝基化合物在角质形成细胞内通过黄素酶硫氧还蛋白还原酶形成的羟基胺的主要代谢物之一。进一步的代谢转化在使用4-氧-2,2,6,6-四甲基哌啶-1-氧基和4-羟基-2,2,6,6-四甲基哌啶-1-氧基的苯甲酸盐作为底物时被检测到。
Metabolism of different nitroxides with piperidine structure used as spin labels in electron spin resonance (ESR) studies in vitro and in vivo was investigated in human keratinocytes of the cell line HaCaT by GC and GC-MS technique combined with S-band ESR. Besides the well known reduction of the nitroxyl radicals to the ESR silent hydroxylamines as primary products our results indicate the formation of the corresponding secondary amines. These reductions are inhibited by the thiol blocking agent N-ethylmaleimide and by the strong inhibitors of the thioredoxin reductase (TR) 2-chloro-2,4-nitrobenzene and 2,6-dichloroindophenol. The competitive inhibitor TR inhibitor azelaic acid and the cytochrome P-450 inhibitor metyrapone lack any effects. The rates of reduction to the hydroxylamines and secondary amines were dependent on the lipid solubility of the nitroxides. Therefore, it can be assumed that the nitroxides must enter the cells for their bioreduction. The mostly discussed intracellular nitroxide reducing substances ascorbic acid and glutathione were unable to form the secondary amines. In conclusion, our results suggest that the secondary amine represents one of the major metabolites of nitroxides besides the hydroxylamine inside keratinocytes formed via the flavoenzyme thioredoxin reductase most probably. Further metabolic conversions were detected with 4-oxo-2,2,6,6-tetramethylpiperidine-1-oxyl and the benzoate of 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl as substrates.
来源:Hazardous Substances Data Bank (HSDB)