1,2-二碘-4,5-二甲基苯 | 5182-67-2
中文名称
1,2-二碘-4,5-二甲基苯
中文别名
——
英文名称
1,2-diiodo-4,5-dimethyl-benzene
英文别名
4,5-diiodo-o-xylene;4,5-diiodo-1,2-dimethylbenzene;1,2-Diiodo-4,5-dimethylbenzene
CAS
5182-67-2
化学式
C8H8I2
mdl
——
分子量
357.961
InChiKey
LXOHTTLTWLSPNR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):4
-
重原子数:10
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2903999090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3,4-二甲基碘苯 3,4-dimethyliodobenzene 31599-61-8 C8H9I 232.064
反应信息
-
作为反应物:描述:1,2-二碘-4,5-二甲基苯 在 potassium permanganate 作用下, 以 吡啶 、 2,2,2-三氟乙醇 为溶剂, 反应 192.0h, 以82%的产率得到4,5-二碘邻苯二甲酸参考文献:名称:六硫代-亚酞菁的简便合成,三重态性质和电化学摘要:鉴于对各种光电器件的高效三重态光敏剂和光敏组件的需求不断增长,我们在此报告六碘代亚酞菁(I 6 SubPcs)的合成和性能。据报道,经过改进的五步合成4,5-二碘代邻苯二甲腈的方法可作为I 6 SubPcs的合成前体。改进的合成仅需进行一次色谱分离即可得到高纯度的目标产物。由于存在六个重碘原子,在I 6 SubPcs中诱导了非常理想的光物理和光化学性质。特别地,单线态氧量子产率(高值Φ Δ测定)为0.83〜0.9。我6个SubPcs研究证明是磷光在77K的2-MeTHF与发射带的最大值(λ P位于)λ = 957和970纳米。激发态-三重态态能量(Ë Ť)被估计为约1.30电子伏特,而三线态寿命(τ Ť)被发现是27.7和30.1微秒。CV / DPV测量表明,这两个I 6 SubPcs均表现出一种不可逆的氧化作用和一种准可逆的还原作用。光谱电化学测量表明,电化学形成的阴离子自由基,即I 6DOI:10.1002/chem.201803316
-
作为产物:描述:参考文献:名称:乙炔插入开放链前驱体中合成六氢三苯并[ a,e,i ] [12]环戊烯摘要:通过在Sonogashira偶联条件下将乙炔插入开链二碘代前体中,可以简单合成六氢三苯并[ a,e,i ] [12]环戊烯,并将其用于制备刚性三臂星形连接器,以作为二维六边形氢键阵列的基本单元。DOI:10.1021/jo202016v
文献信息
-
一种硫酚与邻二碘苯的反应方法
-
Dibenzopentalenes from B(C<sub>6</sub>F<sub>5</sub>)<sub>3</sub>-Induced Cyclization Reactions of 1,2-Bis(phenylethynyl)benzenes作者:Chao Chen、Marcel Harhausen、René Liedtke、Kathrin Bussmann、Aiko Fukazawa、Shigehiro Yamaguchi、Jeffrey L. Petersen、Constantin G. Daniliuc、Roland Fröhlich、Gerald Kehr、Gerhard ErkerDOI:10.1002/anie.201300871日期:2013.6.3'Lene' and mean: The strong Lewis acid B(C6F5)3 efficiently converts some bis(arylethynyl)benzenes into dibenzopentalenes through a series of Lewis acid induced cyclization reactions at room temperature. Thus the reaction has the potential to be useful in the synthesis of substituted dibenzopentalene derivatives which are difficult to make by conventional means.
-
The Direct Iodination of Polyalkylbenzenes Bearing Bulky Groups作者:Hitomi Suzuki、Kiyomi Nakamura、Ryozo GotoDOI:10.1246/bcsj.39.128日期:1966.1The following polyalkylbenzenes bearing bulky groups have been subjected to direct iodination using iodine and periodic acid, and the corresponding mono-iodinated products have been obtained in fairly high yields: 4-t-butyl-1, 2-dimethylbenzene, 5-t-butyl-1, 3-dimethylbenzene, 5-t-butyl-1, 2, 3-trimethylbenzene, 1, 2, 4, 5-tetraisopropylbenzene and p-di-t-butylbenzene. The polyiodination of polymethylbenzenes has also proceeded smoothly, giving polyiodo compounds in high yields. A comparison of various direct methods has proved the above reagent to be the best iodinating agent for a variety of polyalkylbenzenes.
-
Palladium on Carbon: An Efficient, Heterogeneous and Reusable Catalytic System for Carbonylative Synthesis of<i>N</i>-Substituted Phthalimides作者:Mayur V. Khedkar、Shoeb R. Khan、Dinesh N. Sawant、Dattatraya B. Bagal、Bhalchandra M. BhanageDOI:10.1002/adsc.201100460日期:2011.12application of palladium on carbon (Pd/C) as a heterogeneous recyclable catalyst was investigated for the double carbonylation of o-dihaloarenes with amines providing excellent yield of N-substituted phthalimides in shorter reaction time as compared to earlier reported homogeneous protocols. Furthermore, the scope of the developed protocol was applied for the synthesis N-substituted phthalimides from o-halobenzoates
-
Gold Vinylidene Complexes: Intermolecular C(sp<sup>3</sup>)H Insertions and Cyclopropanations Pathways作者:A. Stephen K. Hashmi、Marcel Wieteck、Ingo Braun、Matthias Rudolph、Frank RomingerDOI:10.1002/anie.201204015日期:2012.10.15Highly reactive gold vinylidene species are used for intermolecular C(sp3)H insertions into unactivated alkanes (see scheme). In addition, they can be regarded as synthons for alkylidene carbenes. Initiated by cyclopropanation of the vinylidene species/alkylidene carbenoide, cyclobutene derivatives are formed in a diastereoselective fashion by a ring‐enlargement cascade in only one step.
表征谱图
-
氢谱1HNMR
-
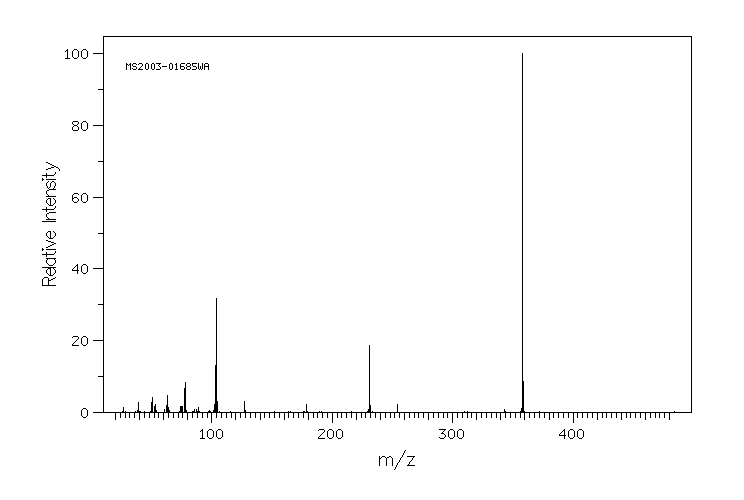
质谱MS
-
碳谱13CNMR
-
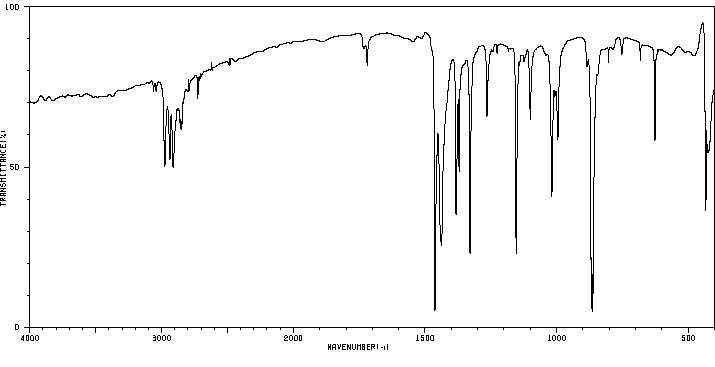
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫