1,1,1-trimethyl-N-phenyl-N-(trimethylsilyl)-silanamine | 4147-89-1
中文名称
——
中文别名
——
英文名称
1,1,1-trimethyl-N-phenyl-N-(trimethylsilyl)-silanamine
英文别名
N,N-Bis(trimethylsilyl)anilin;N,N-bis(trimethylsilyl)-aniline;N-phenyl-N-(trimethylsilyl)silanamine;Silanamine, 1,1,1-trimethyl-N-phenyl-N-(trimethylsilyl)-;N,N-bis(trimethylsilyl)aniline
CAS
4147-89-1
化学式
C12H23NSi2
mdl
——
分子量
237.492
InChiKey
UUBHRQZKROMHOC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:16.5°C
-
密度:0.8951
计算性质
-
辛醇/水分配系数(LogP):4.16
-
重原子数:15
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:3.2
-
氢给体数:0
-
氢受体数:1
安全信息
-
海关编码:2931900090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,1,1-三甲基-N-苯基哌啶 N-trimethylsilylaniline 3768-55-6 C9H15NSi 165.31
反应信息
-
作为反应物:描述:参考文献:名称:有机硅还原剂对硝基芳烃进行无金属脱氧和还原二烯化反应摘要:使用N,N'-双(三甲基甲硅烷基)-4,4'-联吡啶亚烷基(1)在温和和中性的反应条件下实现了硝基芳烃的无金属脱氧和还原二甲硅烷基化反应,并且该反应可能具有广泛的官能团耐受性。单脱氧可得到合成上有价值的N,O-双(三甲基甲硅烷基)苯羟胺(7 a),是一种容易获得且安全的硝基苯来源的苯基亚硝酸,双脱氧可得到N,N-双(三甲基硅烷基)苯胺8。改变1的量很容易控制反应温度以及加入二苯并噻吩(DBTP)。2-芳基硝基苯与1的反应通过N,O-双(三甲基甲硅烷基)苯基羟胺7的热解衍生的原位生成的亚苯基硝基苯胺生成相应的咔唑14,随后将其插入分子内CH。此外,分子内的N-N偶联反应将2,2'-二硝基联苯衍生物还原1,得到相应的苯并[ c ]喹啉。DOI:10.1002/chem.201801972
-
作为产物:描述:偶氮苯 在 三氯化铁 lithium 作用下, 以 四氢呋喃 为溶剂, 反应 23.0h, 生成 1,1,1-trimethyl-N-phenyl-N-(trimethylsilyl)-silanamine参考文献:名称:偶氮化合物的还原性三烷基甲硅烷基化合成双(三烷基甲硅烷基)胺摘要:已经发现,在作为催化剂的 THF 中的过渡金属卤化物存在下,用三烷基氯硅烷和锂的系统还原偶氮化合物可得到双(三烷基甲硅烷基)胺。通过使用叔丁基二甲基氯硅烷作为三烷基氯硅烷显着改变了反应过程。DOI:10.1246/cl.1987.153
-
作为试剂:描述:1-己炔 、 偶氮苯 在 mer-VCl3(tetrahydrofuran)3 、 1,1,1-trimethyl-N-phenyl-N-(trimethylsilyl)-silanamine 作用下, 反应 20.0h, 以56%的产率得到1,2,4-三丁基苯参考文献:名称:双(亚氨基)钒(V)催化的炔烃和偶氮苯的[2+2+1]偶联生成多取代吡咯摘要:VCl3(THF)3 和 N,N-双(三甲基硅基)苯胺 (1a) 的组合是炔烃和偶氮苯的 [2+2+1] 偶联反应的有效催化剂,生成多取代吡咯。一个合理的反应机制涉及生成单(亚氨基)钒(III)物质作为引发步骤,其中 1a 作为亚氨基源,同时释放 2 当量的 ClSiMe3,然后与偶氮苯反应形成催化活性物质通过 N=N 键断裂形成双(亚氨基)钒(V)物质。DOI:10.1021/jacs.8b13390
文献信息
-
Migration of trimethylsilyl group in the reaction of sodium bis(trimethylsilyl)amide with bromobenzene作者:A. V. Lis、I. P. Tsyrendorzhieva、A. I. Albanov、V. I. Rakhlin、M. G. VoronkovDOI:10.1134/s1070428013100084日期:2013.10The reaction of sodium bis(trimethylsilyl)amide with bromobenzene gave a mixture of N,N-bis-(trimethylsilyl)aniline and N,2-bis(trimethylsilyl)aniline, the latter being a rearrangement product formed via 1,3-migration of trimethylsilyl group from the nitrogen atom to the ortho-carbon atom in the benzene ring.
-
Efficient Synthesis of 1,2,4-Dithiazolidine-3,5-diones [Dithiasuccinoyl-Amines] from Bis(chlorocarbonyl)disulfane Plus Bis(trimethylsilyl)amines作者:Michael J. Barany、Robert P. Hammer、R. B. Merrifield、George BaranyDOI:10.1021/ja0455446日期:2005.1.1dithiasuccinoyl (Dts)-amine, serves as a readily removable amino protecting group for building blocks used in syntheses of peptides, glycopeptides, and PNA; it is also useful as a masked isocyanate and (inversely) as a sulfurization reagent for trivalent phosphorus. Bis(chlorocarbonyl)disulfane, the two-sulfur analogue of succinyl chloride, has been envisioned as a reagent for facile single-step elaboration of the
-
1,2-Eliminations in a Novel Reductive Coupling of Nitroarenes to Give Azoxy Arenes by Sodium Bis(trimethylsilyl)amide作者:Jih Ru Hwu、Asish R. Das、Chia Wei Yang、Jiann-Jyh Huang、Ming-Hua HsuDOI:10.1021/ol050924x日期:2005.7.1[reaction: see text]. Symmetric azoxy arenes were successfully prepared in one step from 2 equiv of the corresponding nitroarenes by use of sodium bis(trimethylsilyl)amide as the deoxygenating agents in THF at 150 degrees C in a sealed tube.
-
The reactions of arsenic halides with some organosilicon compounds of nitrogen and sulphur作者:E.W. Abel、D.A. ArmitageDOI:10.1016/s0022-328x(00)83422-1日期:1966.4Arsenic trichloride reacts with silicon-nitrogen and silicon-sulphur compounds to give the chlorosilane and corresponding arsenic-nitrogen or arsenic-sulphur compound. Phenylarsenic dichloride reacts in a similar way with silicon-sulphur compounds but not with silicon-nitrogen derivatives.
-
Insertion reactions of dialkylaluminium derivatives作者:Tadamori Sakakibara、Tadamichi Hirabayashi、Yoshio IshiiDOI:10.1016/s0022-328x(00)88322-9日期:1972.12Five types of reactions between (dimethylalumino)(trimethylsilyl)methylamine Me2AlNMeSiMe3 and carbonyl compounds (ketones, esters and amides) were found to occur, depending on the structure of the carbonyl compounds. Among others two new interesting compounds, 4-(dimethylamino)-4-(methylamino)-3-buten-2-one CH3COCHC(NHMe)NMe2 from acetamide and N, O-dimethylacylimidatetetramethyldialumoxane
表征谱图
-
氢谱1HNMR
-
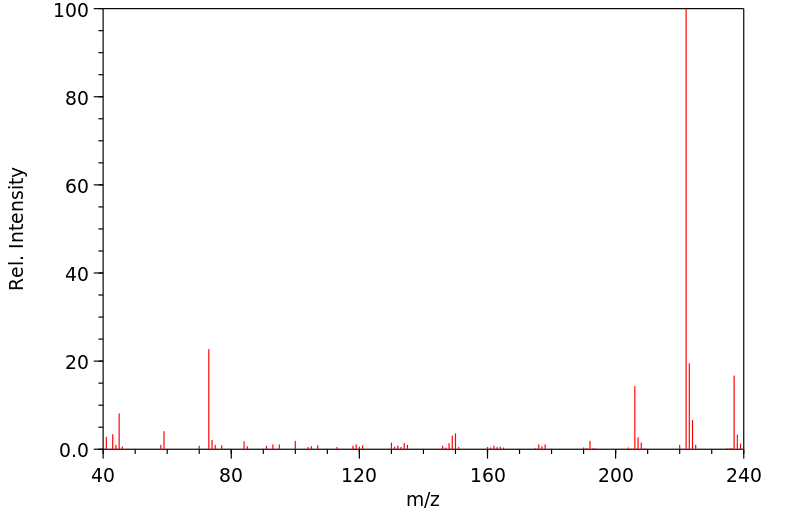
质谱MS
-
碳谱13CNMR
-
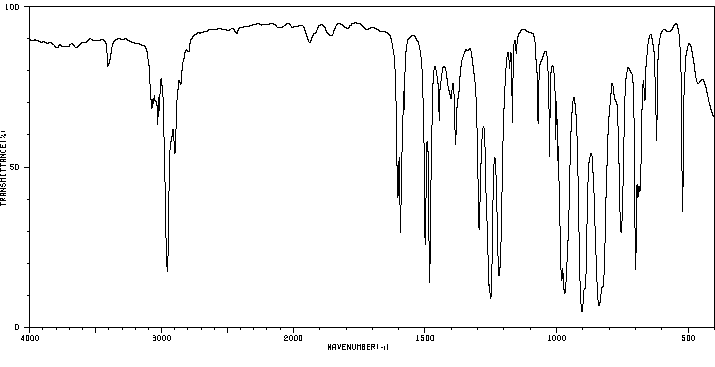
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫