灭幼脲 | 35367-38-5
中文名称
灭幼脲
中文别名
氟脲杀;1-(4-氯苯基)-3-(2,6-二氟苯甲酰基)脲;二氟阻甲脲;除虫脲;敌灭灵;伏虫脲;1-(4-氯苯基)-3-(2,6-二氯苯甲酰基)脲
英文名称
1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea
英文别名
diflubenzuron;N-[(4-chlorophenyl)carbamoyl]-2,6-difluorobenzamide
CAS
35367-38-5
化学式
C14H9ClF2N2O2
mdl
MFCD00055343
分子量
310.687
InChiKey
QQQYTWIFVNKMRW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:230-232°C
-
密度:1.4301 (estimate)
-
溶解度:DMF:10 mg/mL,DMSO:10 mg/mL,DMSO:PBS (pH 7.2) (1:1):0.5 mg/mL
-
物理描述:Diflubenzuron appears as colorless to yellow crystals. Used as a selective insecticide.
-
颜色/状态:Colorless crystals
-
蒸汽压力:9X10-10 mm Hg at 25 °C /gas saturation method/
-
稳定性/保质期:
-
分解:When heated to decomp it emits very toxic fumes of NOx and Cl-.
-
腐蚀性:Non-corrosive
-
碰撞截面:160.98 Ų [M+H]+ [CCS Type: TW]
计算性质
-
辛醇/水分配系数(LogP):3.9
-
重原子数:21
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:58.2
-
氢给体数:2
-
氢受体数:4
ADMET
代谢
埃及棉铃虫对苯甲酰苯基脲类杀虫剂(包括二氟苯脲和三氟苯脲)的解毒机制进行了研究,包括这些杀虫剂在活体内外对水解酶的穿透、降解和抑制速率。研究结果与这些化合物的毒性联系起来。三氟苯脲对埃及棉铃虫第四龄幼虫的毒性比二氟苯脲高10倍。丙氟磷和S,S,S-三丁基硫代磷酸酯对二氟苯脲和三氟苯脲都有增效作用,这表明埃及棉铃虫的主要解毒途径是通过水解。胡椒基丁氧基化合物的有限增效作用表明,混合功能氧化酶在苯甲酰苯基脲的解毒中只起到了相对较小的作用。在埃及棉铃虫中,二氟苯脲的代谢速度比三氟苯脲快:根据幼虫体内母体化合物的相对量,提取出的放射性碳大约有58%是不变的二氟苯脲,而二氟苯脲只有38%。在排泄物中,未变化的三氟苯脲比代谢物排泄得慢(42%以母体化合物形式回收),而二氟苯脲中79%的总提取物以母体化合物形式存在。用亚致死剂量的丙氟磷预处理第四龄幼虫,显著降低了代谢,二氟苯脲比三氟苯脲更明显。在活体内,二氟苯脲代谢抑制与毒性之间存在正相关关系。在体外实验中,所有组织提取物对二氟苯脲的水解速度都比三氟苯脲快。丙氟磷在体外抑制二氟苯脲的水解比三氟苯脲更有效,这由较低的I50值和更陡峭的斜率表明。结果表明,减少穿透和快速消除未变化的(14)C-二氟苯脲以及主要通过水解的快速代谢,是埃及棉铃虫对二氟苯脲解毒的防御机制。
The mechanisms of detoxification of the benzoylphenylureas, diflubenzuron, and teflubenzuron in the Egyptian cotton leafworm, Spodoptera littoralis, were examined, as were rates of penetration, degradation, and inhibition of benzoyphenylureas hydrolase(s) both in vivo and in vitro. The results were considered in connection with the toxicity of these compounds. Teflubenzuron was 10 times more toxic than diflubenzuron to fourth instar larvae of spodoptera littoralis. Profenofos and S,S,S-tributylphosphorotrithioate synergized both diflubenzuron and teflubenzuron, indicating that the major route of detoxification in spodoptera littoralis was through hydrolysis. Limited synergism by piperonly butoxide indicated that mixed function oxidase enzymes play a relatively small role in benzoyphenylureas detoxification. ... In Spodoptera littoralis, diflubenzuron was metabolized more rapidly than teflubenzuron: based on the relative amount of parent compound present in the larval body, about 58% of extracted radiocarbon was unchanged teflubenzuron compared to only 38% diflubenzuron. In the excreta, unchanged teflubenzuron was excreted more slowly than the metabolites (42% recovered as parent compound), compared to diflubenzuron in which 79% of the total extract was present as parent compound. Pretreatment of the fourth instar with sublethal doses of profenofos resulted in a significant decrease in metabolism, more so with diflubenzuron than with teflubenzuron. A positive correlation was found between in vivo diflubenzuron metabolism inhibition and toxicity. In in vitro assays, diflubenzuron was hydrolyzed more rapidly than teflubenzuron by all tissue extracts. Profenofos was more effective in inhibiting the hydrolysis in vitro of diflubenzuron compared to teflubenzuron, as indicated by a lower I50 value and steeper slope. /The/ results indicate that reduced penetration and fast elimination of unchanged (14)(C)diflubenzuron together with rapid metabolism, which occurs mainly through hydrolysis, are defense mechanisms which contribute to diflubenzuron detoxification in spodoptera littoralis.
来源:Hazardous Substances Data Bank (HSDB)
代谢
口服治疗一头牛和一只阉割的绵羊后,收集尿液并进行分析。薄层色谱(TLC)显示存在八种标记物质。绵羊尿液中的主要化合物被确认为2,6-二氟苯甲酸和苯甲酸脒类似物。在牛尿液中,2,6-二氟-3-羟基二氟苯甲脒是主要的代谢物。代谢物N-((4-氯-2-羟基苯基)氨基甲酰基)-2,6-二氟苯甲酰胺和N-((4-氯-3-羟基苯基)氨基甲酰基)-2,6-二氟苯甲酰胺在牛和绵羊的尿液中也被观察到,但4-氯苯基脲仅在牛尿液中看到。两种动物的粪便中含有没有完全消化的食物、2,6-二氟-3-羟基二氟苯甲脒、N-((4-氯-2-羟基苯基)氨基甲酰基)-2,6-二氟苯甲酰胺和N-((4-氯-3-羟基苯基)氨基甲酰基)-2,6-二氟苯甲酰胺。在胆汁中,除了未识别的共轭物外,2,6-二氟-3-羟基二氟苯甲脒、N-((4-氯-2-羟基苯基)氨基甲酰基)-2,6-二氟苯甲酰胺和N-((4-氯-3-羟基苯基)氨基甲酰基)-2,6-二氟苯甲酰胺也出现了。将胆汁水溶性代谢物与β-葡萄糖醛酸酶-芳基硫酸酶一起孵化,将约一半的标记物质转化为可有机提取的物质。尽管TLC显示有八种放射性化合物,但均未确定其身份。对牛奶的分析表明存在未改变的二氟苯甲脒、2,6-二氟苯甲酰胺、2,6-二氟苯甲酸脒和一种未识别的化合物。绵羊和牛的消化液并未显著降解二氟苯甲脒。当代谢物V(2,6-二氟-3-羟基二氟苯甲脒)口服给药给大鼠时,几乎所有的物质在3天内被排出。分析表明存在五种额外的化合物。均未确定其身份。
After oral treatment of a cow and a castrated sheep with labeled diflubenzuron, urine was collected and analyzed. TLC indicated the presence of eight labeled materials. The major compounds in the sheep urine were identified as 2,6-difluorobenzoic acid and the hippurate analog. In the cow's urine, 2,6-difluoro-3-hydroxydiflubenzuron was the major metabolite. Metabolites N-((4-chloro-2-hydroxyphenyl)aminocarbonyl)-2,6-difluorobenzamide and N-((4-chloro-3-hydroxyphenyl)aminocarbonyl)-2,6-difluorobenzamide were also seen in urine of both cow and sheep but the 4-chlorophenylurea was seen only in the urine of the cow. Feces of both animals contained ... 2,6-difluoro-3-hydroxydiflubenzuron, N-((4-chloro-2-hydroxyphenyl)aminocarbonyl)-2,6-difluorobenzamide, and N-((4-chloro-3-hydroxyphenyl)aminocarbonyl)-2,6-difluorobenzamide. In the bile, ... 2,6-difluoro-3-hydroxydiflubenzuron, N-((4-chloro-2-hydroxyphenyl)aminocarbonyl)-2,6-difluorobenzamide, and N-((4-chloro-3-hydroxyphenyl)aminocarbonyl)-2,6-diflurorbenzamide also appeared in addition to unidentified conjugates. Incubation of bile water-soluble metabolites with b-glucuronidase-aryl sulfatase converted about half the labeled material into organic extractable materials. Although TLC indicated eight radioactive compounds, none were identified. Analysis of milk indicated the presence of unchanged diflubenzuron, 2,6-difluorobenzamide, 2,6-difluorohippuric acid, and an unidentified compound. Digestive fluids of sheep and cattle did not significantly degrade diflubenzuron. When metabolite V /2,6-difluoro-3-hydroxydiflubenzuron/ was orally administered to rats, almost all of the material was excreted within 3 days. Analyses indicated the presence of five additional compounds. None were identified.
来源:Hazardous Substances Data Bank (HSDB)
代谢
在大鼠口服5毫克用H3标记在苯甲酰部分和用C14标记在苯胺部分的敌百虫后,95%的H3和70-75%的C14放射性活度在尿液和粪便中被回收。2,6-DFBA被证明构成了超过一半的尿液代谢物。在大鼠呼出的气体中,最高可回收口服剂量1%的5毫克C14-敌百虫标记在苯甲酰部分。
After oral admin to rats of 5 mg diflubenzuron labeled with H3 in the benzoyl and with C14 in the aniline moiety, 95% of the H3 and 70-75% of the C14 radioactivity were retrieved in urine and feces. 2,6-DFBA was shown to constitute more than half of the urinary metabolites. Up to 1% of an oral dose of 5 mg C14-diflubenzuron labeled at the benzoyl moiety was recovered in the expired air of rats.
来源:Hazardous Substances Data Bank (HSDB)
代谢
敌百虫局部应用于成年马蝇和家蝇。马蝇只代谢了大约2%的敌百虫,而家蝇的代谢量约为10%。这两种蝇的代谢在质量上也不同。马蝇提取物中含有2,6-二氟苯甲酰胺和4-氯乙酰苯胺。家蝇提取物中不含有这些物质。家蝇中发现了两种未识别的代谢物,但在马蝇中没有发现。除了这些,4-氯苯脲和一个未知化合物在两种蝇中都有观察到。
Diflubenzuron was applied topically to adult stable flies and houseflies. Stable flies metabolized only about 2% of the diflubenzuron while in houseflies this amounted to about 10%. Metabolism in the two flies differed qualitatively as well. Extracts of stable flies contained 2,6-difluorobenzamide and 4-chloroacetanilide. Extracts of houseflies did not contain these. Two unidentified metabolites were seen in house flies but not stable flies. In addition to these, 4-chlorophenylurea and one unknown compound were observed in both flies.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
diflubenzuron的一种代谢物PCA是近致癌物。它与致癌物结合,可离子化并与DNA反应形成加合物,导致脾肿瘤形成。
One of the metabolites of diflubenzuron, PCA, is a proximate carcinogen. It is conjugated to form the carcinogen that can ionize and reat with DNA to form adducts which result in splenic tumor formation.
来源:Toxin and Toxin Target Database (T3DB)
毒理性
癌症分类:E组 人类非致癌性证据
Cancer Classification: Group E Evidence of Non-carcinogenicity for humans
来源:Hazardous Substances Data Bank (HSDB)
毒理性
对氯苯胺可能对人类有致癌性(2B组)。
para-Chloroaniline is possibly carcinogenic to humans (Group 2B). (L135)
来源:Toxin and Toxin Target Database (T3DB)
毒理性
One of the metabolites of diflubenzuron, PCA, has severe health effects. It is associated with cancer of the spleen and liver and osteosarcomas in male rats. Another metabolite of diflubenzuron also has carcinogenic potential and the same carcinogenic potency as PCA.
来源:Toxin and Toxin Target Database (T3DB)
毒理性
职业性肝毒素 - 第二性肝毒素:在职业环境中的毒性效应潜力是基于人类摄入或动物实验的中毒案例。
Occupational hepatotoxin - Secondary hepatotoxins: the potential for toxic effect in the occupational setting is based on cases of poisoning by human ingestion or animal experimentation.
来源:Haz-Map, Information on Hazardous Chemicals and Occupational Diseases
吸收、分配和排泄
肠道吸收与给药剂量密切相关——剂量越高,相对而言,未改变地在粪便中排出的量就越多。
The intestinal absorption is strongly related to the dosage administered - the higher the dosage, the more is (relatively) excreted unchanged in the feces.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
口服给药后,diflubenzuron被吸收,广泛代谢,并在牛和羊体内几乎完全排泄...在高剂量给药后,会有微量的杀幼虫剂随牛奶排出。
Following oral administration, diflubenzuron is absorbed, extensively metabolized, and almost totally excreted by cattle and sheep ... Trace amounts of the larvicide are excreted in milk following high dosages.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
尼罗罗非鱼鱼苗被暴露于昆虫生长抑制剂diflubenzuron 21天。在实验开始时,将diflubenzuron引入鱼苗所在的养鱼缸中,然后在1、7、14和21天后检测水中、鳃和肝脏中的diflubenzuron水平。当鱼苗保持在2.5和5毫克/升的环境浓度下时,鱼体内积累的diflubenzuron分别是水中含量的76倍和99倍,这表明其生物累积潜力较低。在肝脏和水中发现了一些diflubenzuron的降解产物。
Orechromis niloticus fingerlings were exposed to the insect growth inhibitor diflubenzuron for 21 days. Diflubenzuron was introduced to the aquariums where fish were maintained at the beginning of the experiment, then its level in water, gills and liver was detected after 1, 7, 14 and 21 days. The fish accumulated diflubenzuron 76 and 99 times greater than the water content when kept in an ambient concentration of 2.5 and 5 mg/L, respectively, indicating a low bioaccumulation potential. Some degradation products of diflubenzuron were found mainly in liver and water.
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
危险等级:9
-
危险品标志:C,N
-
安全说明:S60,S61
-
危险类别码:R50/53
-
WGK Germany:2
-
危险品运输编号:3077
-
RTECS号:YS6200000
-
海关编码:2924299031
-
包装等级:III
-
危险类别:9
-
储存条件:| 0-6°C |
SDS
| 第一部分:化学品名称 |
| 化学品中文名称: | 除虫脲;1-(4-氯苯基)-3-(2,6-二氟苯甲酰基)脲 |
| 化学品英文名称: | Diflubenzuron;1-(4-Chlorophenyl)-3-(2,6-difluorobenzoyl)urea |
| 中文俗名或商品名: | |
| Synonyms: | |
| CAS No.: | 35367-38-5 |
| 分子式: | C 14 H 9 ClF 2 N 2 O 2 |
| 分子量: | 310.70 |
| 第二部分:成分/组成信息 |
| 纯化学品 混合物 | ||||||
| 化学品名称:除虫脲;1-(4-氯苯基)-3-(2,6-二氟苯甲酰基)脲 | ||||||
|
| 第三部分:危险性概述 |
| 危险性类别: | 第6.1类 毒害品 |
| 侵入途径: | 吸入 食入 经皮吸收 |
| 健康危害: | 本品为低毒杀虫剂。动物实验,对兔眼睛和皮肤没有刺激作用。资料报道有致突变作用。受热分解放出氯、氟和氮氧化物。 |
| 环境危害: | 对环境有危害,对水体可造成污染。 |
| 燃爆危险: | 本品可燃,具刺激性。 |
| 第四部分:急救措施 |
| 皮肤接触: | 用肥皂水及清水彻底冲洗。就医。 |
| 眼睛接触: | 拉开眼睑,用流动清水冲洗15分钟。就医。 |
| 吸入: | 迅速脱离现场至空气新鲜处。保持呼吸道通畅。如呼吸困难,给输氧。如呼吸停止,立即进行人工呼吸。就医。 |
| 食入: | 误服者,饮适量温水,催吐。就医。 |
| 第五部分:消防措施 |
| 危险特性: | 遇明火、高热可燃。其粉体与空气可形成爆炸性混合物, 当达到一定浓度时, 遇火星会发生爆炸。受高热分解放出有毒的气体。 |
| 有害燃烧产物: | 一氧化碳、二氧化碳、氮氧化物、氯化氢、氟化氢。 |
| 灭火方法及灭火剂: | 泡沫、干粉、砂土。 |
| 消防员的个体防护: | 消防人员须佩戴防毒面具、穿全身消防服,在上风向灭火。 |
| 禁止使用的灭火剂: | |
| 闪点(℃): | |
| 自燃温度(℃): | |
| 爆炸下限[%(V/V)]: | |
| 爆炸上限[%(V/V)]: | |
| 最小点火能(mJ): | |
| 爆燃点: | |
| 爆速: | |
| 最大燃爆压力(MPa): | |
| 建规火险分级: |
| 第六部分:泄漏应急处理 |
| 应急处理: | 隔离泄漏污染区,周围设警告标志,建议应急处理人员戴好防毒面具,穿化学防护服。用砂土或其它不燃性吸附剂混合吸收,然后收集运至废物处理场所。也可以用大量水冲洗,经稀释的洗水放入废水系统。如大量泄漏,收集回收或无害处理后废弃。 |
| 第七部分:操作处置与储存 |
| 操作注意事项: | 密闭操作,局部排风。防止粉尘释放到车间空气中。操作人员必须经过专门培训,严格遵守操作规程。建议操作人员佩戴自吸过滤式防尘口罩,戴化学安全防护眼镜,穿防毒物渗透工作服,戴橡胶手套。远离火种、热源,工作场所严禁吸烟。使用防爆型的通风系统和设备。避免产生粉尘。避免与氧化剂、碱类接触。配备相应品种和数量的消防器材及泄漏应急处理设备。倒空的容器可能残留有害物。 |
| 储存注意事项: | 储存于阴凉、通风的库房。远离火种、热源。防止阳光直射。包装密封。应与氧化剂、碱类分开存放,切忌混储。配备相应品种和数量的消防器材。储区应备有合适的材料收容泄漏物。 |
| 第八部分:接触控制/个体防护 |
| 最高容许浓度: | 中 国 MAC:未制订标准前苏联 MAC:未制订标准美国TLV—TWA:未制订标准 |
| 监测方法: | |
| 工程控制: | 生产过程密闭,加强通风。 |
| 呼吸系统防护: | 空气中粉尘浓度超标时,必须佩戴自吸过滤式防尘口罩。紧急事态抢救或撤离时,应该佩戴空气呼吸器。 |
| 眼睛防护: | 戴安全防护眼镜。 |
| 身体防护: | 穿防毒物渗透工作服。 |
| 手防护: | 戴橡胶手套。 |
| 其他防护: | 工作现场禁止吸烟、进食和饮水。工作后,淋浴更衣。保持良好的卫生习惯。 |
| 第九部分:理化特性 |
| 外观与性状: | 纯品为白色结晶,原药为白色至浅黄色结晶粉末。 |
| pH: | |
| 熔点(℃): | 230~232 |
| 沸点(℃): | |
| 相对密度(水=1): | 1.56 |
| 相对蒸气密度(空气=1): | |
| 饱和蒸气压(kPa): | <1.3×(10-5)/50℃ |
| 燃烧热(kJ/mol): | |
| 临界温度(℃): | |
| 临界压力(MPa): | |
| 辛醇/水分配系数的对数值: | |
| 闪点(℃): | |
| 引燃温度(℃): | |
| 爆炸上限%(V/V): | |
| 爆炸下限%(V/V): | |
| 分子式: | C 14 H 9 ClF 2 N 2 O 2 |
| 分子量: | 310.70 |
| 蒸发速率: | |
| 粘性: | |
| 溶解性: | 难溶于水,易溶于多数有机溶剂。 |
| 主要用途: | 农用杀虫剂。 |
| 第十部分:稳定性和反应活性 |
| 稳定性: | 在常温常压下 稳定 |
| 禁配物: | 强氧化剂、强碱。 |
| 避免接触的条件: | |
| 聚合危害: | 不能出现 |
| 分解产物: | 一氧化碳、二氧化碳、氮氧化物、氟化氢、氯化氢。 |
| 第十一部分:毒理学资料 |
| 急性毒性: | LD50:4640 mg/kg(大鼠经口);2000 mg/kg(兔经皮);>10000 mg/kg(大鼠经皮);4640 mg/kg(小鼠经口) LC50:无资料 |
| 急性中毒: | |
| 慢性中毒: | |
| 亚急性和慢性毒性: | |
| 刺激性: | |
| 致敏性: | |
| 致突变性: | |
| 致畸性: | |
| 致癌性: |
| 第十二部分:生态学资料 |
| 生态毒理毒性: | |
| 生物降解性: | |
| 非生物降解性: | |
| 生物富集或生物积累性: |
| 第十三部分:废弃处置 |
| 废弃物性质: | |
| 废弃处置方法: | 建议用焚烧法处置。在能利用的地方重复使用容器或在规定场所掩埋。 |
| 废弃注意事项: |
| 第十四部分:运输信息 |
| |
| 危险货物编号: | 61904 |
| UN编号: | 2588 |
| 包装标志: | |
| 包装类别: | |
| 包装方法: | 塑料袋或二层牛皮纸袋外全开口或中开口钢桶;两层塑料袋或一层塑料袋外麻袋、塑料编织袋、乳胶布袋;塑料袋外复合塑料编织袋(聚丙烯三合一袋、聚乙烯三合一袋、聚丙烯二合一袋、聚乙烯二合一袋);塑料袋或二层牛皮纸袋外普通木箱;螺纹口玻璃瓶、塑料瓶、复合塑料瓶或铝瓶外普通木箱;塑料瓶、两层塑料袋或两层牛皮纸袋(内或外套以塑料袋)外瓦楞纸箱。 |
| 运输注意事项: | 铁路运输时包装所用的麻袋、塑料编织袋、复合塑料编织袋的强度应符合国家标准要求。运输前应先检查包装容器是否完整、密封,运输过程中要确保容器不泄漏、不倒塌、不坠落、不损坏。严禁与酸类、氧化剂、食品及食品添加剂混运。运输时运输车辆应配备相应品种和数量的消防器材及泄漏应急处理设备。运输途中应防曝晒、雨淋,防高温。公路运输时要按规定路线行驶,勿在居民区和人口稠密区停留。 |
| RETCS号: | |
| IMDG规则页码: |
| 第十五部分:法规信息 |
| 国内化学品安全管理法规: | 化学危险物品安全管理条例 (1987年2月17日国务院发布),化学危险物品安全管理条例实施细则 (化劳发[1992] 677号),工作场所安全使用化学品规定 ([1996]劳部发423号)等法规,针对化学危险品的安全使用、生产、储存、运输、装卸等方面均作了相应规定;常用危险化学品的分类及标志 (GB 13690-92)将该物质划为第6.1 类毒害品。 |
| 国际化学品安全管理法规: |
| 第十六部分:其他信息 |
| 参考文献: | 1.周国泰,化学危险品安全技术全书,化学工业出版社,1997 2.国家环保局有毒化学品管理办公室、北京化工研究院合编,化学品毒性法规环境数据手册,中国环境科学出版社.1992 3.Canadian Centre for Occupational Health and Safety,CHEMINFO Database.1998 4.Canadian Centre for Occupational Health and Safety, RTECS Database, 1989 |
| 填表时间: | 年月日 |
| 填表部门: | |
| 数据审核单位: | |
| 修改说明: | |
| 其他信息: | 5 |
| MSDS修改日期: | 年月日 |
制备方法与用途
杀虫剂除虫脲是一种高效的白色晶体,主要用于防治多种害虫。其化学名称为2,6-二氟-N-[3-(三氯甲基)-4-硝基苯基]苯甲酰胺。它属于苯甲酰脲类杀虫剂,通过抑制昆虫壳多糖的合成,使昆虫不能正常蜕皮或变态而死亡。
物理与化学性质- 纯品为白色晶体,熔点230~232℃。
- 相对密度1.56,蒸气压1.32×10^-5Pa(50℃)。易溶于乙腈、二甲基亚砜,可溶于醋酸乙酯、乙醇、二氯甲烷,稍溶于乙醚、苯、石油醚,在丙酮中溶解度为6.5g/L,在水中为0.1mg/L。
- 对光、热较稳定,常温下贮存稳定,遇碱或强酸易分解,能被土壤微生物分解。工业品为白色至浅黄色结晶,熔点210~230℃。
除虫脲以胃毒作用为主,兼有触杀作用,残效期较长但药效速度较慢。主要用来防治鳞翅目多种害虫如小菜蛾、斜纹夜蛾、甜菜夜蛾、菜青虫等,在卵孵盛期至1~2龄幼虫盛发期使用25%悬浮剂500~1000倍液喷雾。此外,还可以用于玉米、小麦上的粘虫防治及梨小食心虫、毒蛾、松毛虫和稻纵卷叶螟等。
生产方法 安全性除虫脲对人体和环境相对安全。对天敌的影响较小,并且其在动物体内的蓄积作用不明显,能较快代谢,在实验条件下未见致突变、致畸或致癌作用。2年饲喂实验无作用剂量大鼠40mg/L,小鼠50mg/L。对鲑鱼30天饲喂实验LC50为0.3mg/L。对蜜蜂毒性低,急性接触LD50>100μg/头;对鸟类毒性低,8天饲喂试验,野鸭、鹌鹑急性经口LD50>4640mg/L。
上下游信息
反应信息
-
作为反应物:参考文献:名称:WIMMER, MARY J.;SMITH, ROBERT R.;JONES, JEFFREY P., J. AGR. AND FOOD CHEM., 39,(1991) N, C. 280-286摘要:DOI:
-
作为产物:描述:1-(4-chlorophenyl)-3-(3,5-difluorobenzyl)urea 在 10-甲基-9-均三甲苯基吖啶高氯酸盐 作用下, 以 氯仿 为溶剂, 反应 4.0h, 以64%的产率得到灭幼脲参考文献:名称:氢键通过光氧化还原催化胺的需氧氧化†摘要:据报道,在有机光催化剂的催化下,H键相互作用可指导胺的α-C–H氧化为酰胺和氨基酮。带有脲基团的吡咯烷,二芳基胺和苄基胺的好氧氧化具有高收率和广泛的底物范围,证明了该方法的高效率。DOI:10.1039/c8cc06603e
文献信息
-
[EN] ACC INHIBITORS AND USES THEREOF<br/>[FR] INHIBITEURS DE L'ACC ET UTILISATIONS ASSOCIÉES
-
[EN] BICYCLYL-SUBSTITUTED ISOTHIAZOLINE COMPOUNDS<br/>[FR] COMPOSÉS ISOTHIAZOLINE SUBSTITUÉS PAR UN BICYCLYLE申请人:BASF SE公开号:WO2014206910A1公开(公告)日:2014-12-31The present invention relates to bicyclyl-substituted isothiazoline compounds of formula (I) wherein the variables are as defined in the claims and description. The compounds are useful for combating or controlling invertebrate pests, in particular arthropod pests and nematodes. The invention also relates to a method for controlling invertebrate pests by using these compounds and to plant propagation material and to an agricultural and a veterinary composition comprising said compounds.本发明涉及公式(I)中变量如索权和说明中所定义的自行车基取代异噻唑啉化合物。这些化合物对抗或控制无脊椎动物害虫,特别是节肢动物害虫和线虫方面具有用途。该发明还涉及一种通过使用这些化合物来控制无脊椎动物害虫的方法,以及包含所述化合物的植物繁殖材料、农业和兽医组合物。
-
[EN] AZOLINE COMPOUNDS<br/>[FR] COMPOSÉS AZOLINE申请人:BASF SE公开号:WO2015128358A1公开(公告)日:2015-09-03The present invention relates to azoline compounds of formula (I) wherein A, B1, B2, B3, G1, G2, X1, R1, R3a, R3b, Rg1 and Rg2 are as defined in the claims and the description. The compounds are useful for combating or controlling invertebrate pests, in particular arthropod pests and nematodes. The invention also relates to a method for controlling invertebrate pests by using these compounds and to plant propagation material and to an agricultural and a veterinary composition comprising said compounds.本发明涉及式(I)的噁唑啉化合物,其中A、B1、B2、B3、G1、G2、X1、R1、R3a、R3b、Rg1和Rg2如权利要求和描述中所定义。这些化合物对抗或控制无脊椎动物害虫,特别是节肢动物害虫和线虫方面具有用途。该发明还涉及一种利用这些化合物控制无脊椎动物害虫的方法,以及包括所述化合物的植物繁殖材料、农业和兽医组合物。
-
[EN] MICROBIOCIDAL OXADIAZOLE DERIVATIVES<br/>[FR] DÉRIVÉS D'OXADIAZOLE MICROBIOCIDES申请人:SYNGENTA PARTICIPATIONS AG公开号:WO2017157962A1公开(公告)日:2017-09-21Compounds of the formula (I) wherein the substituents are as defined in claim 1, useful as a pesticides, especially fungicides.式(I)的化合物,其中取代基如权利要求1所定义,作为杀虫剂特别是杀菌剂有用。
-
Thieno-pyrimidine compounds having fungicidal activity
表征谱图
-
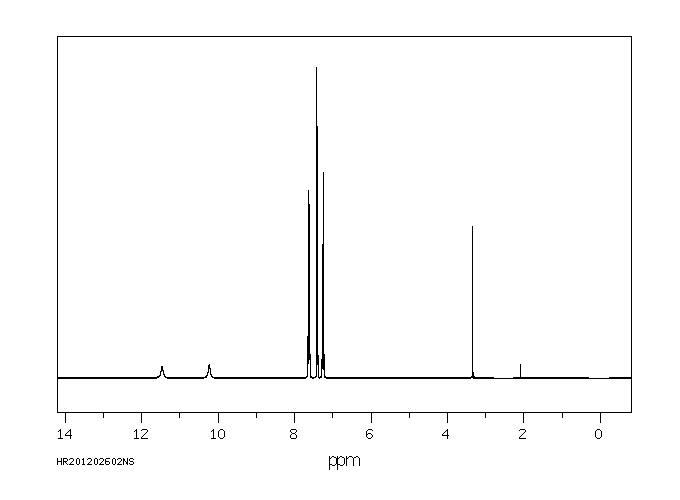
氢谱1HNMR
-
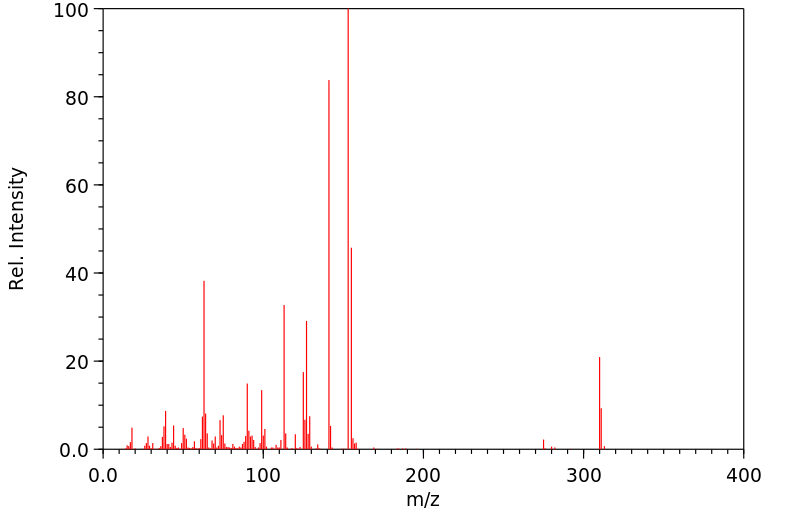
质谱MS
-
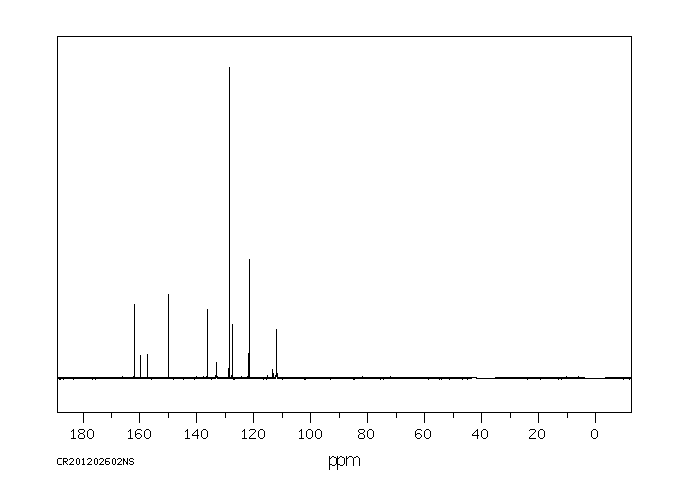
碳谱13CNMR
-
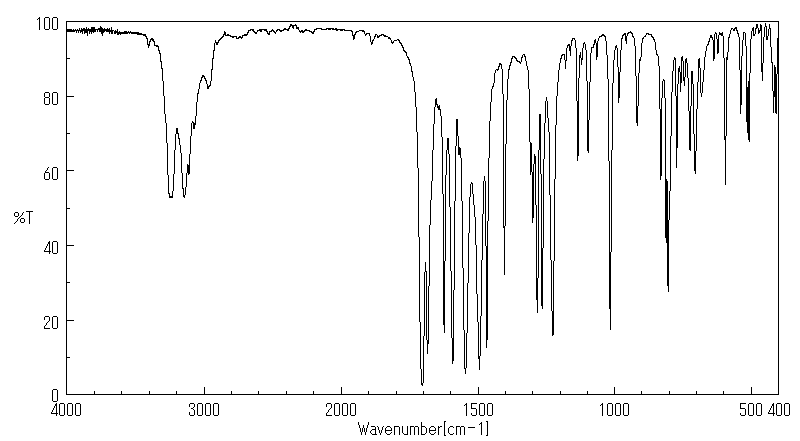
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫