異丁醯溴 | 2736-37-0
中文名称
異丁醯溴
中文别名
异丁酰溴;α-甲基丙酸溴
英文名称
isobutyryl bromide
英文别名
2-methylpropanoyl bromide
CAS
2736-37-0
化学式
C4H7BrO
mdl
——
分子量
151.003
InChiKey
ICNCZFQYZKPYMS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:117°C
-
密度:1,41 g/cm3
-
稳定性/保质期:
遵照规定使用和储存,则不会发生分解。
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:6
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险等级:8/3
-
安全说明:S16,S26,S36,S37,S39
-
危险类别码:R10
-
危险品运输编号:UN 2924
-
储存条件:存放在阴凉干燥处
SDS
异丁酰溴 修改号码:5
模块 1. 化学品
产品名称: Isobutyryl Bromide
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第3级
金属腐蚀性 第1级
健康危害
皮肤腐蚀/刺激 1B类
严重损伤/刺激眼睛 第1级
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 易燃液体和蒸气
可能腐蚀金属
造成严重的皮肤灼伤和眼损伤
防范说明
[预防] 远离热源/火花/明火/热表面。禁烟。
保持容器密闭。
只可存放于原用的容器内。
使用防爆的电气/通风/照明设备。采取预防措施以防静电和火花引起的着火。
切勿吸入。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
异丁酰溴 修改号码:5
模块 2. 危险性概述
[急救措施] 吸入:将受害者移到新鲜空气处,在呼吸舒适的地方保持休息。
食入:漱口。切勿催吐。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
皮肤接触:立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
被污染的衣物清洗后方可重新使用。
立即呼叫解毒中心/医生。
吸收溢出物,防止材料被损坏。
[储存] 存放于通风良好处。保持凉爽。
存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 异丁酰溴
百分比: >95.0%(GC)
CAS编码: 2736-37-0
分子式: C4H7BrO
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
立即呼叫解毒中心/医生。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
立即呼叫解毒中心/医生。
食入: 立即呼叫解毒中心/医生。漱口。切勿引吐。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,二氧化碳
不适用的灭火剂: 水
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:喷水,保持容器冷却,注意切勿直接接触水。如果安全,消除一切火源
。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(自携式呼吸器)。远离溢出物/泄露处并处在上风处。确保
紧急措施: 足够通风。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 回收到密闭容器前用干砂或惰性吸收剂吸收泄漏物。一旦大量泄漏,筑堤控制。附着
物或收集物应该根据相关法律法规废弃处置。
副危险性的防护措施 切勿与水接触。移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防
爆设备。
模块 7. 操作处置与储存
处理
异丁酰溴 修改号码:5
模块 7. 操作处置与储存
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离热源/火花/明火
/热表面。禁烟。采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 如果可能,使用封闭系统。如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
可能产生高压。小心打开。
使用耐腐蚀设备。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
存放于惰性气体环境中。
防湿。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
潮敏
包装材料: 依据法律。只可存放在原用的容器內。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 半面罩或全面罩呼吸器,自携式呼吸器(SCBA),供气呼吸器等。依据当地和政府法
规,使用通过政府标准的呼吸器。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 极淡的黄色-浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 117 °C
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 1.39
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 与水接触分解并产生有毒气体。
避免接触的条件: 火花, 明火, 静电, 湿气
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 溴化氢
模块 11. 毒理学信息
急性毒性: 无资料
异丁酰溴 修改号码:5
模块 11. 毒理学信息
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第8类 腐蚀品
副危险性: 第3类:易燃液体
UN编号: 2920
正式运输名称: 腐蚀性液体, 易燃的, 不另作详细说明
包装等级: II
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: Isobutyryl Bromide
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第3级
金属腐蚀性 第1级
健康危害
皮肤腐蚀/刺激 1B类
严重损伤/刺激眼睛 第1级
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 易燃液体和蒸气
可能腐蚀金属
造成严重的皮肤灼伤和眼损伤
防范说明
[预防] 远离热源/火花/明火/热表面。禁烟。
保持容器密闭。
只可存放于原用的容器内。
使用防爆的电气/通风/照明设备。采取预防措施以防静电和火花引起的着火。
切勿吸入。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
异丁酰溴 修改号码:5
模块 2. 危险性概述
[急救措施] 吸入:将受害者移到新鲜空气处,在呼吸舒适的地方保持休息。
食入:漱口。切勿催吐。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
皮肤接触:立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
被污染的衣物清洗后方可重新使用。
立即呼叫解毒中心/医生。
吸收溢出物,防止材料被损坏。
[储存] 存放于通风良好处。保持凉爽。
存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 异丁酰溴
百分比: >95.0%(GC)
CAS编码: 2736-37-0
分子式: C4H7BrO
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
立即呼叫解毒中心/医生。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
立即呼叫解毒中心/医生。
食入: 立即呼叫解毒中心/医生。漱口。切勿引吐。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,二氧化碳
不适用的灭火剂: 水
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:喷水,保持容器冷却,注意切勿直接接触水。如果安全,消除一切火源
。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(自携式呼吸器)。远离溢出物/泄露处并处在上风处。确保
紧急措施: 足够通风。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 回收到密闭容器前用干砂或惰性吸收剂吸收泄漏物。一旦大量泄漏,筑堤控制。附着
物或收集物应该根据相关法律法规废弃处置。
副危险性的防护措施 切勿与水接触。移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防
爆设备。
模块 7. 操作处置与储存
处理
异丁酰溴 修改号码:5
模块 7. 操作处置与储存
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离热源/火花/明火
/热表面。禁烟。采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 如果可能,使用封闭系统。如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
可能产生高压。小心打开。
使用耐腐蚀设备。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
存放于惰性气体环境中。
防湿。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
潮敏
包装材料: 依据法律。只可存放在原用的容器內。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 半面罩或全面罩呼吸器,自携式呼吸器(SCBA),供气呼吸器等。依据当地和政府法
规,使用通过政府标准的呼吸器。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 极淡的黄色-浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 117 °C
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 1.39
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 与水接触分解并产生有毒气体。
避免接触的条件: 火花, 明火, 静电, 湿气
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 溴化氢
模块 11. 毒理学信息
急性毒性: 无资料
异丁酰溴 修改号码:5
模块 11. 毒理学信息
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第8类 腐蚀品
副危险性: 第3类:易燃液体
UN编号: 2920
正式运输名称: 腐蚀性液体, 易燃的, 不另作详细说明
包装等级: II
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-溴-2-甲基丙酰溴 2-Bromoisobutyryl bromide 20769-85-1 C4H6Br2O 229.899
反应信息
-
作为反应物:描述:参考文献:名称:Kaschirsky, Zhurnal Russkago Fiziko-Khimicheskago Obshchestva, 1881, vol. 13, p. 90,95摘要:DOI:
-
作为产物:描述:参考文献:名称:Brown, Carl E.; Neville, Anthony G.; Rayner, David M., Australian Journal of Chemistry, 1995, vol. 48, # 2, p. 363 - 380摘要:DOI:
文献信息
-
Convergent Catalytic Asymmetric Synthesis of Esters of Chiral Dialkyl Carbinols作者:Ze-Peng Yang、Gregory C. FuDOI:10.1021/jacs.0c01324日期:2020.3.25Because chiral dialkyl carbinols, as well as their derived esters, are significant as intermediates and end points in fields such as organic, pharmaceutical, and biological chemistry, the development of efficient approaches to their asymmetric synthesis is an important endeavor. In this report, we describe a method for the direct catalytic enantioselective synthesis of such esters, beginning with an
-
Insertion of arynes into carbon–halogen σ-bonds: regioselective acylation of aromatic rings作者:Hiroto Yoshida、Yasuhiro Mimura、Joji Ohshita、Atsutaka KunaiDOI:10.1039/b701581j日期:——Arynes were found to insert into carbonâhalogen Ï-bonds of various acid halides, enabling acyl and halogen moieties to be introduced simultaneously into adjacent positions of aromatic rings.氮烯能够插入各种酰卤中的碳-卤素σ键,从而使得酰基和卤素基团同时引入到芳香环的相邻位置。
-
Reactions of CH bonds in organica oxygenates with octaethylporphyrinato rhodium(II) and iridium(II) dimers作者:Kenneth J. Del Rossi、Xiao-Xiang Zhang、Bradford B. WaylandDOI:10.1016/0022-328x(95)05569-b日期:1995.11Metalloporphyrin dimers, [(OEP)M]2 (), are observed to react with CH bond units in aldehyes, kenotes and benzyl alohols. Aldehydes, R2CHCHO, preferentially react at the alkyl group adjacent to the carbonyl position to yield β-formyl complexes, (OEP)M-CR2CHO, even when reaction of the aldehydic CH unit is thermodynamically favored. Reaction at the carbon-hydrogen bond adjacent to the carbonyl group观察到金属卟啉二聚体[(OEP)M] 2()与醛,酮和苄醇中的CH键单元反应。醛,R 2 CHCHO优先在与羰基位置相邻的烷基上反应,生成β-甲酰基络合物(OEP)M-CR 2 CHO,即使在热力学上有利于醛CH单元的反应。有人提出,通过将金属卟啉加到烯醇异构体中以生成中间体烷基桥连的配合物(OEP)M-CR'(OH)-M(OEP),可以在与羰基相邻的碳氢键上发生反应。随后通过有效的β-OH迁移反应形成(OEP)M-CR 2 C(O)R'和(OEP)MH复合物。[(OEP)Rh] 2也与苄醇C反应6 H 5 CH(OH)R(R = H,CH 3,C(O)C 6 H 5),制得中间体α-氢烷基衍生物(OEP)Rh-CR(OH)C 6 H 5,随后离解得到有机羰基物质,C 6 H [in5] C(O)R和(OEP)Rh-H。
-
Indium- and Zinc-Mediated Acyloxyallylation of Protected and Unprotected Aldotetroses—Revealing a Pronounced Diastereodivergence and a Fundamental Difference in the Performance of the Mediating Metal作者:Markus Draskovits、Christian Stanetty、Ian R. Baxendale、Marko D. MihovilovicDOI:10.1021/acs.joc.7b03063日期:2018.3.2were successfully applied to the indium and also zinc-mediated acyloxyallylation, with the latter being a first for an unprotected sugar. The investigation largely benefited from the choice of these more exotic starting materials as it allowed unambiguous identification/quantification of the hexose-products which are available as authentic reference materials. The observed diastereoselectivities indicate十多年前,未经保护的醛糖的酰氧基化首次被证实为还原糖的潜在的优雅的二碳同系物(通过臭氧分解)。但是,它在实际案例合成中的应用仍然很少。在如此成功的展示之后,我们回答了有关这一有吸引力的转变的几个悬而未决的问题,我们进行了深入的方法学重新调查。将未受保护和受保护形式的差向异构体四聚体l-赤藓糖和d-苏糖成功应用于铟以及锌介导的酰氧基烯丙基化,后者是未保护糖的首创。该研究很大程度上得益于这些更具异国情调的起始原料的选择,因为它可以对己糖产品进行明确的鉴定/定量,而后者可作为可靠的参考材料。观察到的非对映选择性表明对底物有很强的控制作用(O2处的立体化学),并详细研究了试剂结构对选择性的影响。相关保护和未保护结构之间存在强烈的面部非对映差异,并且铟和锌之间的性能表现出乎意料的显着差异。
-
Lewis Acid–Lewis Base-Catalysed Enantioselective Addition of α-Ketonitriles to Aldehydes作者:Stina Lundgren、Erica Wingstrand、Christina MobergDOI:10.1002/adsc.200600365日期:2007.2.5Additions of structurally diverse α-ketonitriles to aromatic and aliphatic prochiral aldehydes yielding highly enantioenriched acylated cyanohydrins were achieved using a combination of a titanium salen dimer and an achiral or chiral Lewis base. In most cases high yields and high enantioselectivities were observed. The ee was moderate in the initial part of the reaction but increased over time. This
表征谱图
-
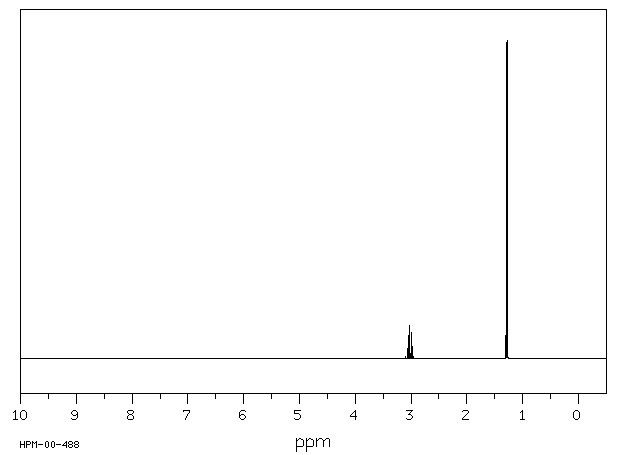
氢谱1HNMR
-
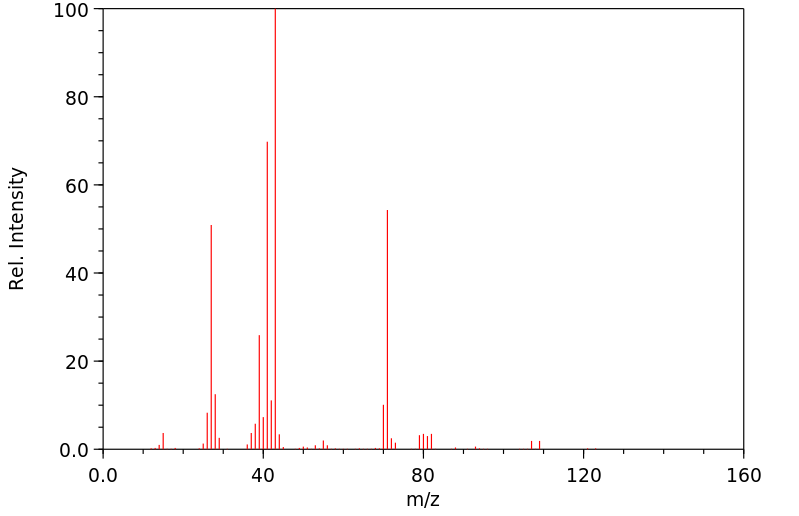
质谱MS
-
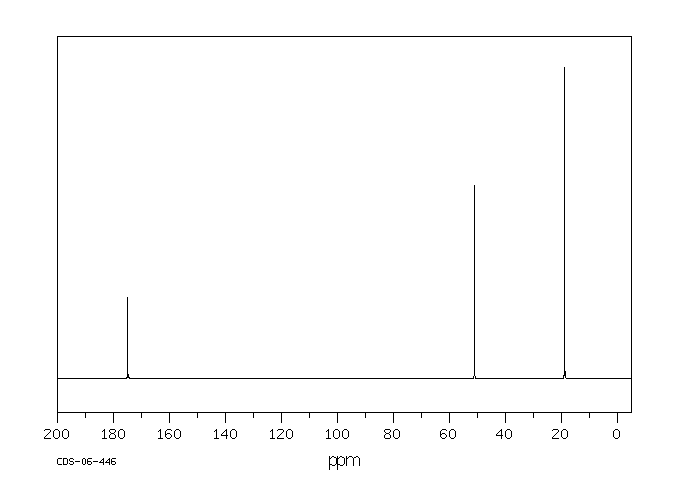
碳谱13CNMR
-
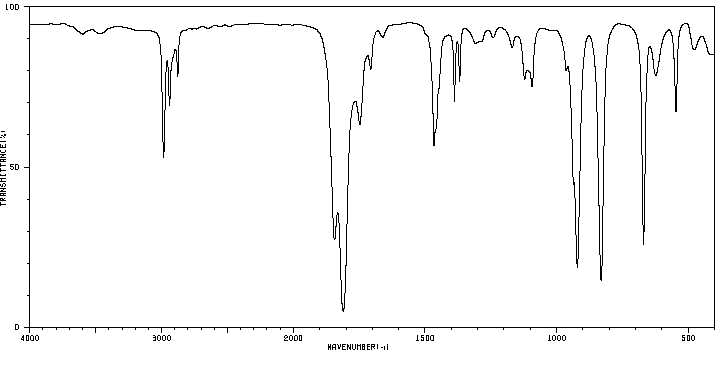
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-六氢-3a(1H)-并环戊二烯羰基氯化物
金刚烷酰氯
辛酰溴
辛酰氯
辛酰氟
辛-5t-烯酰氯
衣康酰氯
螺[3.5]壬烷-2-甲酰氯
螺[3.4]辛烷-2-甲酰氯
草酰溴
草酰氯
草酰氟
花生酰氯
花生四烯酰氯
肉豆蔻酰氯
肉豆蔻酰-1-13C氯
癸酰氯
癸基二酰二氯
癸二酰氯
異丁醯溴
甲酰氯
甲酰基溴化物
甲氧基乙酰氯
甲基丙烯酰氯
甲基丙烯酰氟
环辛烷羰基氯化物
环戊烷羰基溴
环戊烷羰基氟
环戊基甲酰氯
环戊基乙酰氯
环戊基乙酰氯
环庚烷羰酰氯
环己酰溴
环己甲酰氯
环己基乙酰氯
环己-3-烯-1-甲酰氯
环丙烷乙酰氯
环丙烷丙酰氯
环丁烷羰基碘化物
环丁烷羰基溴化物
环丁烷-1,2-二甲酰氯
环丁基甲酰氯
溴二氯乙酰氯
溴乙酰溴
溴乙酰氯
溴(二氟)乙酰氯
油酰氯
氰基乙酰溴
氯碘乙酰氯
氯氟乙酰氟