甲基肼 | 60-34-4
中文名称
甲基肼
中文别名
1-甲基-肼;一甲基肼;甲肼;一甲肼;甲基联胺
英文名称
methylhydrazine
英文别名
Monomethylhydrazine;N-methylhydrazine
CAS
60-34-4
化学式
CH6N2
mdl
——
分子量
46.072
InChiKey
HDZGCSFEDULWCS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
稳定性/保质期:
-
遇明火、高热会引起燃烧爆炸。接触空气能自燃或干燥品久贮后会变质并可能自燃。
-
稳定性良好,无特别注意事项([24])。
-
应避免与强氧化剂、氧及过氧化物接触([25])。
-
避免受热([26])。
-
不会发生聚合反应([27])。
-
分解产物为氨([28])。
-
计算性质
-
辛醇/水分配系数(LogP):-0.9
-
重原子数:3
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:38
-
氢给体数:2
-
氢受体数:2
ADMET
代谢
甲基肼通过纯化的鼠肝微粒体混合功能胺氧化酶催化的N-氧化反应如下所示。在pH 7.7和25°C条件下,甲基肼的N-氧化最大速率与二甲苯胺几乎相同。尽管可以检测到甲烷作为甲基肼N-氧化的产物,但它可能代表了一种N-氧化中间体的化学分解产物。
The n-oxidation of methylhydrazine catalyzed by purified mouse liver microsomal mixed function amine oxidase is shown. At pH 7.7 and 25 °C, methylhydrazine has nearly the same maximal n-oxidation rate as dimethylaniline. Although methane can be detected as a product of n-oxidation of methylhydrazine, it may represent the chemical decomposition product of a n-oxidized intermediate.
来源:Hazardous Substances Data Bank (HSDB)
代谢
Flavoprotein n-oxygenase... catalyzes formation of... n-oxides of methylhydrazine /and/ 1,1-dimethylhydrazine... .
来源:Hazardous Substances Data Bank (HSDB)
代谢
分离的肝细胞和肝微粒体与偏二甲肼、1,1-二甲基肼和1,2-二甲基肼一起孵化产生了自由基中间体,这些中间体通过电子自旋共振光谱法使用4-吡啶基-1-氧化物-t-丁基硝酮作为自旋捕捉剂被检测到。来自这三种肼衍生物的旋加合物的光谱特征与4-吡啶基-1-氧化物-t-丁基硝酮甲基自由基加合物的报告值相符。在微粒体准备中,混合功能氧化酶系统的抑制剂以及通过用氯化钴预处理大鼠来破坏细胞色素P450均减少了自由基的形成。甲巯咪唑,一种含有FAD的单加氧酶系统的抑制剂,同样减少了1,1-二甲基肼的活化,但并未减少偏二甲肼和1,2-二甲基肼的活化。向肝微粒体中加入生理浓度的谷胱甘肽(GSH)使ESR信号的强度降低了大约80%。与此一致,将分离的肝细胞与甲基肼一起孵化降低了细胞内GSH的含量,这表明GSH可以有效清除甲基自由基。获得的结果表明甲基自由基可能是负责甲基肼的毒性/或致癌作用的烷基化物种。
Isolated hepatocytes and liver microsomes incubated with monomethylhydrazine, 1,1-dimethylhydrazine and 1,2-dimethylhydrazine produced free radical intermediates which were detected by ESR spectroscopy by using 4-pyridyl-1-oxide-t-butyl nitrone as spin trapping agent. The spectral features of the spin adducts derived from all three hydrazine derivatives corresponded to the values reported for the methyl free radical adduct of 4-pyridyl-1-oxide-t-butyl nitrone. In the microsomal preparations, inhibitors of the mixed function oxidase system and the destruction of cytochrome P450 by pretreating the rats with cobalt chloride all decreased the free radical formation. Methimazole, an inhibitor of FAD-containing monoxygenase system, similarly decreased the activation of 1,1-dimethylhydrazine, but not that of monomethylhydrazine and 1,2-dimethylhydrazine. The addition to liver microsomes of physiological concentrations of glutathione (GSH) lowered by approx 80% the intensities of the ESR signals. Consistently, incubation of isolated hepatocytes with methylhydrazine decreased the intracellular GSH content, suggesting that GSH can effectively scavenge the methyl free radicals. The results obtained suggest that methyl free radicals could be the alkylating species responsible for the toxic and/or carcinogenic effect of methylhydrazines.
来源:Hazardous Substances Data Bank (HSDB)
代谢
甲基肼可以通过大鼠肝脏切片代谢成二氧化碳。检测到了能够与核酸共价结合的反应性代谢物。甲基肼的生物转化可能不是一个解毒过程,而可能产生自身具有活性的代谢物。在给予最高可行剂量的甲基肼的情况下,仓鼠的肝脏DNA鸟嘌呤没有被甲基化。这看起来不像是氢化物处理动物中看到的DNA鸟嘌呤甲基化的重要中间体。在肝脏微粒体、氧气和能量产生辅因子的存在下,单烷基肼(包括甲基肼)被转化为相应的烃。肝脏中的混合功能氨基氧化酶可以在氧气和NADPH的存在下氧化烷基肼。甲基肼形成甲烷可能是由于N-氧化中间体的分解。...甲基肼被中性粒细胞(来自大鼠腹膜渗出物)氧化,导致形成烷基自由基。叠氮化物可以抑制这一步骤。
Methylhydrazine can be metabolized to carbon dioxide by rat liver slices. Reactive metabolites that were capable of binding covalently to nucleic acids were detected. Biotransformation of methylhydrazine might not be a detoxification process but may produce metabolites that are themselves active. No liver DNA guanine could be methylated in hamsters given the highest feasible doses of methylhydrazine. This does not appear to be an important intermediate in the methylation of DNA guanine seen in hydrazine-treated animals. Monoalkylhydrazines, including methylhydrazine, were converted to the corresponding hydrocarbons in the presence of liver microsomes, oxygen, and energy-producing co-factors. Mixed-function aminooxidases in the liver can oxidize alkylhydrazines in the presence of oxygen and NADPH. The formation of methane from methylhydrazine may be due to the decomposition of an N-oxidation intermediate. ...Methylhydrazine is oxidized by neutrophils (from rat peritoneal exudates) leading to the formation of alkyl radicals. Azide could inhibit this step.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
A3; 已确认的动物致癌物,对人类的相关性未知。
A3; Confirmed animal carcinogen with unknown relevance to humans.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
根据荷兰社会事务与就业部长的要求,荷兰卫生委员会评估了工作场所物质的致癌性质,并提出了一个参考欧盟指令的分类。这种评估由荷兰职业标准专家委员会执行。现在的报告包含了该委员会对N-甲基肼致癌性的评估。委员会得出结论,N-甲基肼应被视为对人类有致癌性(与欧盟2类相似)。N-甲基肼具有基因毒性。评估。没有关于人类的数据。有足够的证据表明N-甲基肼在实验动物中具有致癌性。吸入N-甲基肼在小鼠和仓鼠中诱发了良性和恶性肿瘤,口服(饮用水)暴露在一个实验中导致小鼠发生良性肿瘤,仓鼠发生恶性肿瘤。在大鼠和狗吸入后没有发现肿瘤,但大鼠的暴露时间可能太短,即1年而不是OECD指南451推荐的2年。有一些证据表明在体外细菌系统中具有突变活性。在哺乳动物细胞系统中没有诱导突变,但已经发现染色体和DNA损伤。在体内,N-甲基肼在大鼠和小鼠的显性致死试验和狗的微核试验中为阴性。在使用碱性洗脱技术评估体内肝脏DNA损伤时,得到了相互矛盾的结果。分类建议。委员会认为N-甲基肼应被视为对人类有致癌性。它被归类为一种基因毒性致癌物(分类与欧盟2类相似)。
At request of the Minister of Social Affairs and Employment, the Health Council of the Netherlands evaluates the carcinogenic properties of substances at the workplace and proposes a classification with reference to the EU-directive. This evaluation is performed by the Dutch Expert Committee on Occupational Standards. The present report contains an evaluation by the committee on the carcinogenicity of N-methylhydrazine. The Committee concludes that N-methylhydrazine should be considered as carcinogenic to humans (comparable with EU-category 2). N-methylhydrazine is genotoxic. Evaluation. No data on humans are available. There is sufficient evidence for the carcinogenicity of N-methylhydrazine in experimental animals. Inhalation of N-methylhydrazine induced benign and malignant tumors in mice and hamsters and oral (drinking water) exposure caused benign tumors in mice and malignant tumors in hamsters in one experiment. No tumors were found in rats and dogs following inhalation, but the exposure time in rats may have been too short, that is 1 year instead of 2 years as recommended in OECD guideline 451. There is some evidence for mutagenic activity in in vitro bacterial systems. No mutations were induced in mammalian cell systems, but chromosome and DNA damage have been found. In vivo, N-methylhydrazine was negative in a dominant lethal assay in rats and mice and in a micronucleus test in dogs. Conflicting results were obtained with respect to DNA damage in liver in vivo assessed with the alkaline elution technique. Recommendation for classification. The committee is of the opinion that N-methylhydrazine should be considered as carcinogenic to humans. It is classified as a genotoxic carcinogen (classification comparable with EU category 2).
来源:Hazardous Substances Data Bank (HSDB)
毒理性
该物质可以通过吸入其蒸汽、通过皮肤接触以及摄入进入人体。
The substance can be absorbed into the body by inhalation of its vapour, through the skin and by ingestion.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
毒理性
吸入,皮肤吸收,吞食,皮肤和/或眼睛接触
inhalation, skin absorption, ingestion, skin and/or eye contact
来源:The National Institute for Occupational Safety and Health (NIOSH)
毒理性
眼睛、皮肤、呼吸系统刺激;呕吐、腹泻、震颤、共济失调;缺氧、发绀;抽搐;[潜在的职场致癌物]
irritation eyes, skin, respiratory system; vomiting, diarrhea, tremor, ataxia; anoxia, cyanosis; convulsions; [potential occupational carcinogen]
来源:The National Institute for Occupational Safety and Health (NIOSH)
吸收、分配和排泄
甲基肼可以通过肺部、消化道、注射部位和皮肤被吸收。
Methylhydrazine is absorbed from the lung, GI tract, injection sites, and skin.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
(14)C labeled methylhydrazine was administered to mice, dogs, and monkeys. All species excreted 25-40% of the dose in urine within 24 hr. In mice ... approx the same amount was excreted in respired air. Both carbon dioxide and radioactive methane were found.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
通过放射性示踪技术研究了大鼠注射单甲基肼(MMH)的呼吸和尿液排泄。给予0.12 MM/kg的大鼠在接下来的24小时内呼吸了大约45%的标记碳。在呼吸的放射性中,20-25%是标记为二氧化碳的(14)C,其余是(14)C-甲烷。在亚惊厥剂量下,40%的注射放射性物质通过尿液排出。从更高剂量的尿液中排出的(14)C百分比较低,但净排出的量略高。
The respiratory and urinary excretion of ip administered monomethylhydrazine (MMH) by rats was studied by means of radiotracer technique. Rats given 0.12 mM/kg respired approx 45% of the labeled carbon during the following 24 hr. Of the respired radioactivity, 20-25% was (14)C labeled carbon dioxide and the remainder was (14)C-methane. At the subconvulsive dose, 40% of the administered radioactivity was excreted in the urine. The percentage of urinary excretion of (14)C from higher doses was less, but the net amount excreted was slightly higher.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
未稀释的甲基肼以14.7-264.5 mg/kg的剂量应用于狗皮肤后,在应用后30秒内在血液中检测到。
Undiluted methyl hydrazine applied to dog skin at doses of 14.7-264.5 mg/kg was detected in the blood within 30 sec following application.
来源:Hazardous Substances Data Bank (HSDB)
制备方法与用途
制备方法
以水合肼和苯甲醛为原料,经缩合、甲基化反应而得。将39%的水合肼加入反应锅内,在20-30℃搅拌下滴加苯甲醛,约7小时加完后继续搅拌2小时,放置过夜。次日过滤,用水洗涤至近中性,烘干得粒状固体苄叉连氮,熔点为90-93℃,收率约为95%以上。
将苯、苄叉连氮和硫酸二甲酯加入反应锅内,在密闭条件下搅拌回流5小时。冷却后析出固体,加水使固体溶解,分层并进行水蒸气蒸馏至无法蒸出苯甲醛为止。然后加入无水乙醇,析出固体,冷却后过滤得到硫酸甲肼。收率为60%-70%。
合成制备方法以水合肼和苯甲醛为原料,经缩合、甲基化反应而得。将39%的水合肼加入反应锅内,在20-30℃搅拌下滴加苯甲醛,约7小时加完后继续搅拌2小时,放置过夜。次日过滤,用水洗涤至近中性,烘干得粒状固体苄叉连氮,熔点为90-93℃,收率约为95%以上。
将苯、苄叉连氮和硫酸二甲酯加入反应锅内,在密闭条件下搅拌回流5小时。冷却后析出固体,加水使固体溶解,分层并进行水蒸气蒸馏至无法蒸出苯甲醛为止。然后加入无水乙醇,析出固体,冷却后过滤得到硫酸甲肼。收率为60%-70%。
用途简介 用途上下游信息
反应信息
-
作为反应物:参考文献:名称:Nitrogenase Model Complexes [Cp*Fe(μ-SR1)2(μ-η2-R2N═NH)FeCp*] (R1 = Me, Et; R2 = Me, Ph; Cp* = η5-C5Me5): Synthesis, Structure, and Catalytic N−N Bond Cleavage of Hydrazines on Diiron Centers摘要:The reactions of [Cp*Fe(mu-SR1)(3)FeCp*] (Cp* = eta(5)-C5Me5; R-1 = Et, Me) with 1.5 equiv (RNHNH2)-N-2 (R-2 = Ph, Me) give the mu-eta(2)-diazene diiron thiolate-bridged complexes [Cp*Fe(mu-SR1)(2)(mu-eta(2)-(RN)-N-2 NH)FeCp*], along with the formation of PhNH2 and NH3. These mu-eta(2)-diazene diiron thiolate-bridged complexes exhibit excellent catalytic N-N bond cleavage of hydrazines under ambient conditions.DOI:10.1021/ja805025w
-
作为产物:参考文献:名称:一种常压下催化合成甲基肼的新方法摘要:本发明公开了一种常压下催化合成甲基肼的新方法。其特征是:将水合肼与一氯甲烷以盐酸作保护剂,硅胶作催化剂,乙醇作溶剂,70~74℃下常压反应,生成甲基肼盐酸盐。采用肼游离方法,通过水合肼游离出甲基肼后,经精馏工艺获得甲基肼水溶液。游离后的副产物一盐酸肼盐可以循环套用。本发明具有如下优点:设备成本和原料价格较为低廉,反应产率高且选择性好,无三废产生,环保性强,工艺实现了内循环,可以方便的进行连续化生产,并且反应在常压下进行,操作简单,生产安全。公开号:CN105037196B
-
作为试剂:描述:肼基甲酸叔丁酯 在 四丁基溴化铵 、 potassium carbonate 、 N,N-二异丙基乙胺 、 甲基肼 、 N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate 作用下, 以 乙醇 、 N,N-二甲基甲酰胺 、 甲苯 、 乙腈 为溶剂, 反应 51.0h, 生成 tert-butyl 2-(4-(2-oxo-2-(phenethylamino)ethyl)benzoyl)-1-propylhydrazine-1-carboxylate参考文献:名称:10.3390/md22060250摘要:DOI:10.3390/md22060250
文献信息
-
Plant Growth Regulator Daminozide Is a Selective Inhibitor of Human KDM2/7 Histone Demethylases作者:Nathan R. Rose、Esther C. Y. Woon、Anthony Tumber、Louise J. Walport、Rasheduzzaman Chowdhury、Xuan Shirley Li、Oliver N. F. King、Clarisse Lejeune、Stanley S. Ng、Tobias Krojer、Mun Chiang Chan、Anna M. Rydzik、Richard J. Hopkinson、Ka Hing Che、Michelle Daniel、Claire Strain-Damerell、Carina Gileadi、Grazyna Kochan、Ivanhoe K. H. Leung、James Dunford、Kar Kheng Yeoh、Peter J. Ratcliffe、Nicola Burgess-Brown、Frank von Delft、Susanne Muller、Brian Marsden、Paul E. Brennan、Michael A. McDonough、Udo Oppermann、Robert J. Klose、Christopher J. Schofield、Akane KawamuraDOI:10.1021/jm300677j日期:2012.7.26N-demethylation of Nε-methyl lysine residues in histones and are current therapeutic targets. A set of human 2-oxoglutarate analogues were screened using a unified assay platform for JmjC demethylases and related oxygenases. Results led to the finding that daminozide (N-(dimethylamino)succinamic acid, 160 Da), a plant growth regulator, selectively inhibits the KDM2/7 JmjC subfamily. Kinetic and crystallographic
-
[EN] CRBN LIGANDS AND USES THEREOF<br/>[FR] LIGANDS CRBN ET LEURS UTILISATIONS申请人:KYMERA THERAPEUTICS INC公开号:WO2019140387A1公开(公告)日:2019-07-18The present invention provides compounds, compositions thereof, and methods of using the same for the inhibition of CRBN, and the treatment of CRBN-mediated disorders.本发明提供了化合物、其组合物以及使用这些化合物抑制CRBN并治疗CRBN介导的疾病的方法。
-
Rh(i) and Ir(i) catalysed intermolecular hydroamination with substituted hydrazines
-
Synthesis of 4-Substituted Chlorophthalazines, Dihydrobenzoazepinediones, 2-Pyrazolylbenzoic Acid, and 2-Pyrazolylbenzohydrazide via 3-Substituted 3-Hydroxyisoindolin-1-ones作者:Hanh Nho Nguyen、Victor J. Cee、Holly L. Deak、Bingfan Du、Kathleen Panter Faber、Hakan Gunaydin、Brian L. Hodous、Steven L. Hollis、Paul H. Krolikowski、Philip R. Olivieri、Vinod F. Patel、Karina Romero、Laurie B. Schenkel、Stephanie D. Geuns-MeyerDOI:10.1021/jo3000628日期:2012.4.20hydrazine, followed by chlorination with POCl3. We have also discovered two novel transformations of 3-vinyl- and 3-alkynyl-3-hydroxyisoindolinones. Addition of vinyl organometallic reagents to N,N-dimethylaminophthalimide (8a) provided dihydrobenzoazepinediones 15a–15c via the proposed ring expansion of 3-vinyl-3-hydroxyisoindolinone intermediates. 3-Alkynyl-3-hydroxyisoindolinones react with hydrazine and在本文中,我们描述了以良好的总收率进行的4-取代的氯邻苯二甲腈的一般三步合成。在关键步骤中,N,N-二甲基氨基邻苯二甲酰亚胺(8a)指导烷基,芳基和杂芳基有机金属试剂的选择性单加成反应,得到3-取代的3-羟基异吲哚满酮9b,9i - 9am。通过与肼反应,然后用POCl 3氯化,许多羟基异吲哚啉酮可转化为氯酞嗪1b - 1v。我们还发现了3-乙烯基和3-炔基-3-羟基异吲哚满酮的两个新颖的转化。将乙烯基有机金属试剂添加到N,N-二甲基氨基邻苯二甲酰亚胺(8a)通过提议的3-乙烯基-3-羟基异吲哚满酮中间体的扩环作用提供了二氢苯并氮杂氮杂二酮15a - 15c。3-炔基-3-羟基异吲哚啉酮与肼和取代的肼反应,生成2-吡唑基苯甲酸16a - 16d和2-吡唑基苯并酰肼17a - 17g,而不是预期的炔基酞菁。
-
Studying Products of Hydrazine Interaction with Isothiocyanates by Means of Chromatography and Mass Spectrometry作者:A. V. Ul’yanov、K. E. Polunin、I. A. Polunina、A. K. BuryakDOI:10.1134/s0036024421050290日期:2021.5compounds in real-time and delayed modes are optimized. The physicochemical characteristics of the sorption of thiosemicarbazides are determined. The decomposition and fragmentation of their metastable protonated molecules are studied. Schemes are proposed for the formation of fragmented and characteristic thiosemicarbazide ions in different modes of ionization.
表征谱图
-
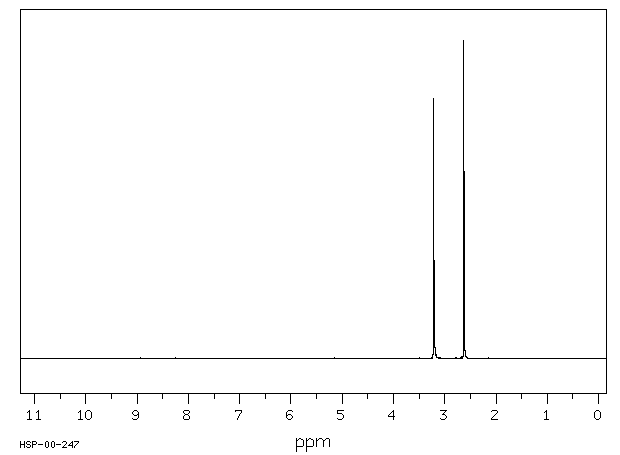
氢谱1HNMR
-
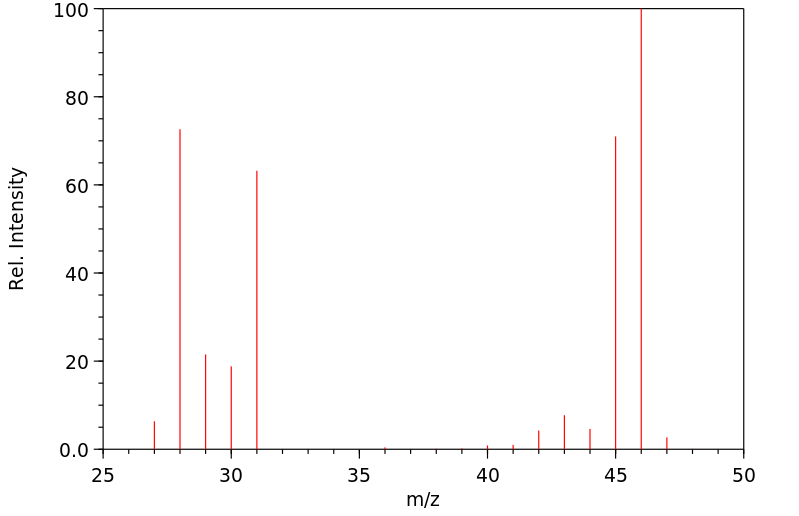
质谱MS
-
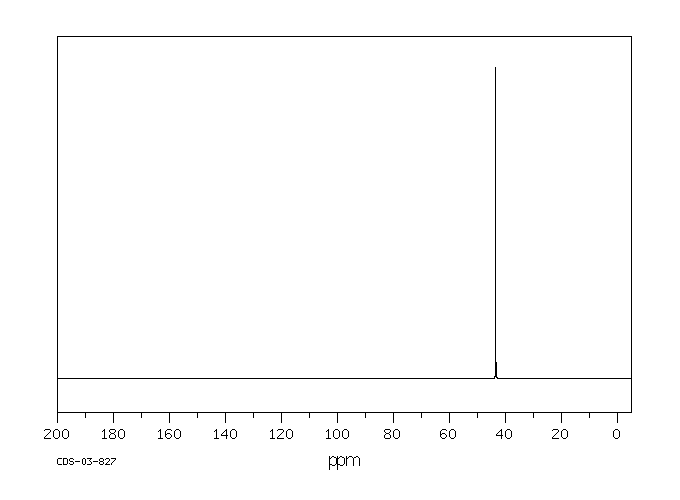
碳谱13CNMR
-
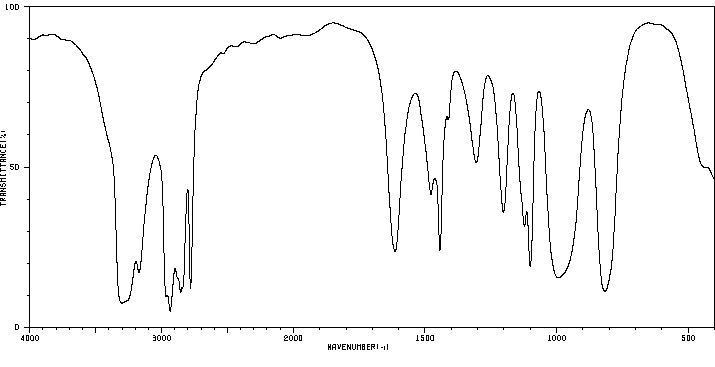
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷