potassium 1,2-naphthoquinone-4-sulphonate | 5908-27-0
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:286-288 °C(lit.)
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):-2.66
-
重原子数:17
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:99.7
-
氢给体数:0
-
氢受体数:5
安全信息
-
危险品标志:Xi
-
安全说明:S22,S26,S37,S39
-
危险类别码:R36/37/38
-
储存条件:请将药品存放在避光、阴凉且干燥的地方,并密封保存。
SDS
| Name: | 1 2-Naphthoquinone-4-sulfonic acid potassi um salt tech. 90% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 5908-27-0 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 5908-27-0 | 1,2-Naphthoquinone-4-sulfonic acid pot | tech., 9 | 227-611-7 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
Causes respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 5908-27-0: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: orange
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 286 - 288 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C10H5KO5S
Molecular Weight: 276.29
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, oxides of sulfur, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 5908-27-0 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1,2-Naphthoquinone-4-sulfonic acid potassi um salt - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
IMO
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 5908-27-0: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 5908-27-0 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 5908-27-0 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
文献信息
-
Tautomerism of 4-amino- and 4-arylamino-1,2-naphthoquinones.作者:HIROSHIGE YANO、MIYUKI YAMASAKI、YUKIKO SHIMOMURA、MASATAKE IWASAKI、MUTSUKO OHTA、YOHKO FURUNO、KEIKI KOUNO、YUKIO ONO、YO UEDADOI:10.1248/cpb.28.1207日期:——The 13C chemical shifts of 4-amino- and 4-arylamino-1, 2-naphthoquinones measured in DMSO-d6, pyridine-d5 and a D2O solution of NaOD indicated that the predominant tautomer in neutral solvents is the 1, 2-dioxo-4-amino-naphthalene (Ia) form, while that in weak basic solvents is the 1-oxo-2-hydroxy-4-imino-naphthalene (Ib) form and that in an aqueous solution of NaOH is the anion of the Ib form. The electronic spectrum of 4-anilino-1, 2-naphthoquinone measured in a mixture of EtOH and 0.05M H3SO4 (1 : 4 by volume) indicated that its tautomeric form in an aqueous strong acid solution is the Ia form and that the compound is hydrolyzed quickly to produce 2-hydroxy-1, 4-naphtho-quinone. The magnitudes of substituent effects observed in the electronic spectra of 4-(4'-substituted)-anilino-1, 2-naphthoquinones measured in EtOH were found to be smaller than those measured in pyridine. This difference was considered to provide confirmation that the predominant tautomer in neutral solvents is the Ia form. The theoretical π-π* transition energies of 4-arylamino-1, 2-naphthoquinones were calculated for both the Ia and the Ib forms by means of Pariser-Parr-Pople type molecular orbital calculations.在DMSO-d6、吡啶-d5和NaOD的D2O溶液中测得的4-氨基和4-芳氨基-1,2-萘醌的13C化学位移表明,在中性溶剂中,主要互变异构体是1,2-二氧代-4-氨基萘(Ia)形式,而在弱碱性溶剂中,是1-氧代-2-羟基-4-亚氨基萘(Ib)形式,在NaOH水溶液中,是Ib形式的阴离子。在EtOH和0.05M H3SO4(体积比1:4)的混合物中测得的4-苯胺基-1,2-萘醌的电子光谱表明,在水性强酸溶液中,其互变异构形式是Ia形式,并且该化合物迅速水解生成2-羟基-1,4-萘醌。发现在EtOH中测得的4-(4'-取代)苯胺基-1,2-萘醌的电子光谱中的取代基效应的幅度小于在吡啶中测得的。这种差异被认为是证实中性溶剂中主要互变异构体为Ia形式的证据。通过Pariser-Parr-Pople型分子轨道计算,分别计算了4-芳氨基-1,2-萘醌的Ia和Ib形式的理论π-π*跃迁能量。
-
Novel pyrazino-phenanthroline based rigid donor-π-acceptor compounds: A detail study of optical properties, acidochromism, solvatochromism and structure-property relationship作者:Shantaram Kothavale、Nagaiyan SekarDOI:10.1016/j.dyepig.2016.08.032日期:2017.1Donor-π-acceptor-π-auxiliary acceptor type compounds have been designed and synthesized to study their rigidity and structural effects on optical properties. Structural variation is achieved by changing the central π-conjugated core with naphthalene, acenaphthene and phenanthrene based donor-π-acceptor compounds containing N, N-diethyl aniline or morpholine as donor and pyrazine ring fused with phenanthroline
-
一种螺噁嗪类光致变色材料申请人:宁波萃英化学技术有限公司公开号:CN108794506A公开(公告)日:2018-11-13本发明公开了一种螺噁嗪类光致变色材料,具有如式1所示结构,本发明初期着色度小,显色有效浓度低,变色灵敏,褪色速度快,耐久性好,无底色。
-
Spectrophotometric determination of sulfanilic acid and sulfonamides in pharmaceutical samples with potassium 1,2-naphthoquinone-4-sulfonate作者:A. Punta Cordero、F. J. Barragán de la Rosa、A. GuiraumDOI:10.1139/v89-244日期:1989.10.1
An accurate and simple method is proposed for the determination of sulfanilic acid in the presence of sulfonamides. This method is based on measuring the intensity of the red colour that develops when sulfanilic acid is allowed to react with potassium 1,2-naphthoquinone-4-sulphonate (NS) in a chloroaceticchloroacetate buffer at pH 3,4. Colour development reaches completion after 2 h, allowing sulfanilic acid to be quantified spectrophotometrically at 470 nm (ε = 4.7 × 103 L mol−1 cm−1). The main product causing colour formation, potassium 1,2-naphthoquinone-4-(N-aminophenylen-4-sulphonate) (NSSA), was isolated and characterized. When samples also contain sulfonamides an extraction into chloroform must be performed. Sulfanilic acid in binary mixtures with sulfanilamide, sulfacetamide, or sulfathiazole can be determined either by direct measurement of the aqueous phase after extraction at 470 nm or by subtracting from the absorbance of the aqueous phase before extraction the absorbance of sulfonamide as determined by measuring the extracted chloroform phase at 345 nm. Sulfadiazine, sulfamethoxipyridazine, and sulfamethoxazole interferences are prevented by their extraction into chloroform at pH 7.2; these species cannot be determined. The effects of pH, reagent concentration, time, and temperature on colour formation were investigated. In all cases the standard addition method gave more accurate results. The method was applied to several pharmaceutical samples. Keywords: spectrophotometry, sulfanilic acid, sulfonamides, potassium 1,2-napthoquinone-4-sulphonate.
提出了一种准确简单的方法,用于在磺胺类药物存在的情况下确定磺胺基苯甲酸。该方法基于测量磺胺基苯甲酸与1,2-萘醌-4-磺酸钾(NS)在pH 3.4的氯乙酸-氯乙酸盐缓冲液中反应时形成的红色强度。颜色发展在2小时后达到完成,可以在470 nm处通过分光光度法定量测量磺胺基苯甲酸(ε = 4.7×103 L mol-1 cm-1)。导致颜色形成的主要产物,1,2-萘醌-4-(N-氨基苯甲酸-4-磺酸钾)(NSSA)被分离和表征。当样品还包含磺胺类药物时,必须进行氯仿萃取。在氯仿相萃取测量345 nm时,可以通过直接测量萃取前的水相的吸光度或通过从水相吸光度中减去测定的磺胺类药物的吸光度来确定二元混合物中的磺胺基苯甲酸、磺胺基乙酰胺或磺胺嘧啶。磺胺嘧啶、磺胺甲氧嘧啶和磺胺甲氧苯嘧啶的干扰可以通过在pH 7.2下将其萃取到氯仿中来防止;这些物种无法确定。研究了pH、试剂浓度、时间和温度对颜色形成的影响。在所有情况下,标准加入法给出了更准确的结果。该方法应用于几种药品样品。关键词:分光光度法、磺胺基苯甲酸、磺胺类药物、1,2-萘醌-4-磺酸钾。 -
BIOSENSOR申请人:MATSUSHITA ELECTRIC INDUSTRIAL CO., LTD.公开号:EP0136362A1公开(公告)日:1985-04-10A measuring electrode (27) and an adjacent electrode (28) are embedded in an insulating substrate (26). The exposed surface of the electrode system is coated with a porous substance (29) carrying an oxidation reductase (30) and, if needed, an electron acceptor (31) in a dry state, to form a biosensor. A predetermined voltage is applied between the electrodes (27, 28) of the biosensor, and a matrix-containing sample is dropped on the porous substance (29). A current proportional to the substrate concentration flows, so it is possible to quantitatively determine the matrix concentration of various biological samples highly accurately, rapidly and easily.
表征谱图
-
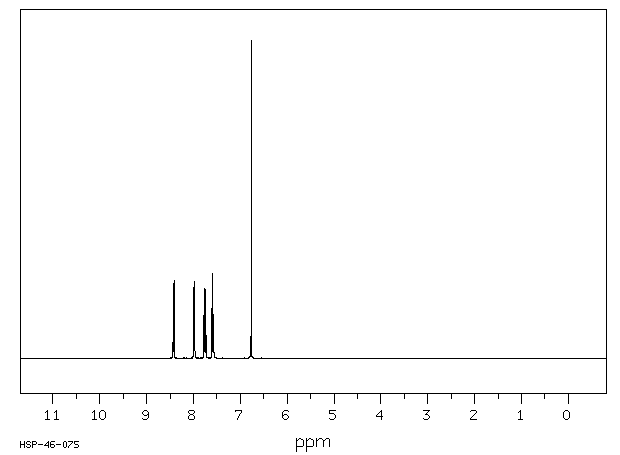
氢谱1HNMR
-
质谱MS
-
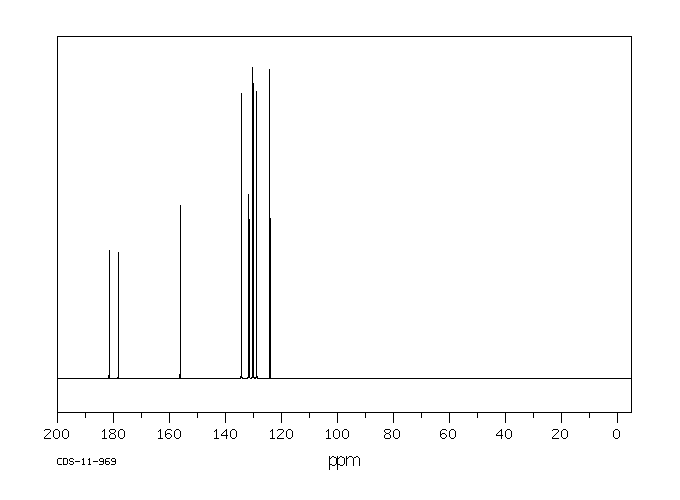
碳谱13CNMR
-
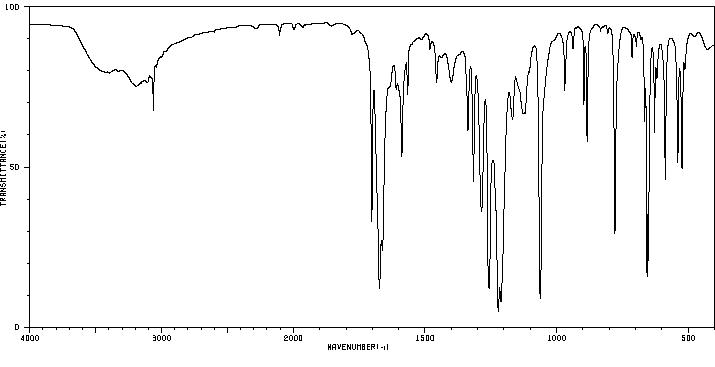
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息