避蚊胺 | 134-62-3
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-45 °C
-
沸点:111 °C1 mm Hg
-
密度:0.998 g/mL at 20 °C(lit.)
-
蒸气密度:6.7 (vs air)
-
闪点:>230 °F
-
溶解度:DMSO(微溶)、乙酸乙酯(微溶)、甲醇(微溶)
-
物理描述:N,n-diethyl-m-toluamide appears as clear colorless or faintly yellow slightly viscous liquid. Faint pleasant odor. (NTP, 1992)
-
颜色/状态:Nearly colorless to amberlike liquid
-
气味:Faint, characteristic odor
-
蒸汽密度:6.7 (NTP, 1992) (Relative to Air)
-
蒸汽压力:0.002 mm Hg at 25 °C
-
稳定性/保质期:
-
分解:When heated to decomposition it emits toxic fumes of /nitrogen oxides/.
-
粘度:13.3 cP at 30 °C
-
折光率:Index of refraction: 1.5212 at 20 °C/D
-
碰撞截面:143.2 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards]
-
保留指数:1571;1571;1583;269.36
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:14
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.416
-
拓扑面积:20.3
-
氢给体数:0
-
氢受体数:1
ADMET
安全信息
-
TSCA:Yes
-
危险等级:6.1(b)
-
危险品标志:Xn
-
安全说明:S61
-
危险类别码:R22,R36/38,R52/53
-
WGK Germany:2
-
海关编码:29242995
-
危险品运输编号:2810
-
危险类别:6.1(b)
-
RTECS号:XS3675000
-
包装等级:III
-
储存条件:使用白铁桶或铝桶进行盛装。
SDS
| 第一部分:化学品名称 |
| 化学品中文名称: | N,N-二乙基间甲苯酰胺 |
| 化学品英文名称: | N,N-Diethyl-m-toluamide;Diethyl-m-toluamide |
| 中文俗名或商品名: | |
| Synonyms: | |
| CAS No.: | 134-62-3 |
| 分子式: | C 12 H 17 NO |
| 分子量: | 191.27 |
| 第二部分:成分/组成信息 |
| 纯化学品 混合物 | |||
| 化学品名称:N,N-二乙基间甲苯酰胺 | |||
|
| 第三部分:危险性概述 |
| 危险性类别: | |
| 侵入途径: | 吸入 食入 经皮吸收 |
| 健康危害: | 本品对皮肤有刺激作用。蒸气或烟雾对眼睛、粘膜和上呼吸道有刺激作用。 |
| 环境危害: | |
| 燃爆危险: | 本品可燃,具刺激性。 |
| 第四部分:急救措施 |
| 皮肤接触: | 脱去污染的衣着,用流动清水冲洗。 |
| 眼睛接触: | 立即翻开上下眼睑,用流动清水冲洗15分钟。就医。 |
| 吸入: | 脱离现场至空气新鲜处。呼吸困难时给输氧。呼吸停止时,立即进行人工呼吸。就医。 |
| 食入: | 误服者漱口,给饮牛奶或蛋清,就医。 |
| 第五部分:消防措施 |
| 危险特性: | 遇高热、明火或与氧化剂接触,有引起燃烧的危险。燃烧分解时,放出有毒的氮氧化物。 |
| 有害燃烧产物: | 一氧化碳、二氧化碳、氧化氮 |
| 灭火方法及灭火剂: | 消防人员须佩戴防毒面具、穿全身消防服,在上风向灭火。尽可能将容器从火场移至空旷处。喷水保持火场容器冷却,直至灭火结束。处在火场中的容器若已变色或从安全泄压装置中产生声音,必须马上撤离。灭火剂:雾状水、泡沫、干粉、二氧化碳、砂土。 |
| 消防员的个体防护: | |
| 禁止使用的灭火剂: | |
| 闪点(℃): | >110 |
| 自燃温度(℃): | 无资料 |
| 爆炸下限[%(V/V)]: | 无资料 |
| 爆炸上限[%(V/V)]: | 无资料 |
| 最小点火能(mJ): | |
| 爆燃点: | |
| 爆速: | |
| 最大燃爆压力(MPa): | |
| 建规火险分级: |
| 第六部分:泄漏应急处理 |
| 应急处理: | 迅速撤离泄漏污染区人员至安全区,并进行隔离,严格限制出入。切断火源。建议应急处理人员戴自给正压式呼吸器,穿防毒服。尽可能切断泄漏源。防止流入下水道、排洪沟等限制性空间。小量泄漏:用大量水冲洗,洗水稀释后放入废水系统。大量泄漏:构筑围堤或挖坑收容。用泵转移至槽车或专用收集器内,回收或运至废物处理场所处置。 |
| 第七部分:操作处置与储存 |
| 操作注意事项: | 密闭操作,全面通风。操作人员必须经过专门培训,严格遵守操作规程。建议操作人员佩戴自吸过滤式防毒面具(半面罩),戴化学安全防护眼镜,穿防毒物渗透工作服,戴橡胶耐油手套。远离火种、热源,工作场所严禁吸烟。使用防爆型的通风系统和设备。防止蒸气泄漏到工作场所空气中。避免与氧化剂、还原剂、酸类接触。搬运时要轻装轻卸,防止包装及容器损坏。配备相应品种和数量的消防器材及泄漏应急处理设备。倒空的容器可能残留有害物。 |
| 储存注意事项: | 储存于阴凉、通风的库房。远离火种、热源。应与氧化剂、还原剂、酸类、食用化学品分开存放,切忌混储。配备相应品种和数量的消防器材。储区应备有泄漏应急处理设备和合适的收容材料。 |
| 第八部分:接触控制/个体防护 |
| 最高容许浓度: | 中 国 MAC:未制订标准前苏联MAC:未制订标准美国TLV—TWA:未制订标准美国 |
| 监测方法: | |
| 工程控制: | 生产过程密闭,全面通风。 |
| 呼吸系统防护: | 可能接触其蒸气时,戴面具式呼吸器。 |
| 眼睛防护: | 戴化学安全防护眼镜。 |
| 身体防护: | 穿防腐工作服。 |
| 手防护: | 戴橡皮胶手套。 |
| 其他防护: | 工作现场严禁吸烟。工作后,淋浴更衣。注意个人清洁卫生。定期体检。 |
| 第九部分:理化特性 |
| 外观与性状: | 无色液体。 |
| pH: | |
| 熔点(℃): | <25 |
| 沸点(℃): | 160(2.5kPa) |
| 相对密度(水=1): | 0.99(20℃) |
| 相对蒸气密度(空气=1): | 6.7 |
| 饱和蒸气压(kPa): | 2.53(160℃) |
| 燃烧热(kJ/mol): | |
| 临界温度(℃): | |
| 临界压力(MPa): | |
| 辛醇/水分配系数的对数值: | |
| 闪点(℃): | >110 |
| 引燃温度(℃): | 无资料 |
| 爆炸上限%(V/V): | 无资料 |
| 爆炸下限%(V/V): | 无资料 |
| 分子式: | C 12 H 17 NO |
| 分子量: | 191.27 |
| 蒸发速率: | |
| 粘性: | |
| 溶解性: | 溶于水,溶于醇、醚。 |
| 主要用途: | 用于有机合成。 |
| 第十部分:稳定性和反应活性 |
| 稳定性: | 在常温常压下 稳定 |
| 禁配物: | 强氧化剂、强还原剂、强酸、强碱。 |
| 避免接触的条件: | |
| 聚合危害: | 不能出现 |
| 分解产物: | 一氧化碳、二氧化碳、氧化氮。 |
| 第十一部分:毒理学资料 |
| 急性毒性: | LD50:1950mg/kg(大鼠经口);3180mg/kg(兔经皮) LC50:5950mg/m3(大鼠吸入) |
| 急性中毒: | |
| 慢性中毒: | |
| 亚急性和慢性毒性: | |
| 刺激性: | |
| 致敏性: | |
| 致突变性: | |
| 致畸性: | |
| 致癌性: |
| 第十二部分:生态学资料 |
| 生态毒理毒性: | |
| 生物降解性: | |
| 非生物降解性: | |
| 生物富集或生物积累性: |
| 第十三部分:废弃处置 |
| 废弃物性质: | |
| 废弃处置方法: | 处置前应参阅国家和地方有关法规。建议用焚烧法处置。焚烧炉排出的氮氧化物通过洗涤器除去。 |
| 废弃注意事项: |
| 第十四部分:运输信息 |
| |
| 危险货物编号: | |
| UN编号: | |
| 包装标志: | |
| 包装类别: | |
| 包装方法: | |
| 运输注意事项: | 运输前应先检查包装容器是否完整、密封,运输过程中要确保容器不泄漏、不倒塌、不坠落、不损坏。严禁与氧化剂、还原剂、酸类、食用化学品等混装混运。运输车船必须彻底清洗、消毒,否则不得装运其它物品。船运时,配装位置应远离卧室、厨房,并与机舱、电源、火源等部位隔离。公路运输时要按规定路线行驶。 |
| RETCS号: | |
| IMDG规则页码: |
| 第十五部分:法规信息 |
| 国内化学品安全管理法规: | |
| 国际化学品安全管理法规: |
| 第十六部分:其他信息 |
| 参考文献: | 1.周国泰,化学危险品安全技术全书,化学工业出版社,1997 2.国家环保局有毒化学品管理办公室、北京化工研究院合编,化学品毒性法规环境数据手册,中国环境科学出版社.1992 3.Canadian Centre for Occupational Health and Safety,CHEMINFO Database.1998 4.Canadian Centre for Occupational Health and Safety, RTECS Database, 1989 |
| 填表时间: | 年月日 |
| 填表部门: | |
| 数据审核单位: | |
| 修改说明: | |
| 其他信息: | 3 |
| MSDS修改日期: | 年月日 |
制备方法与用途
避蚊胺(英文名称 diethyltoluamide,简称DEET),化学名为二乙基甲苯酰胺,是一种广谱昆虫驱避剂,适用于多种叮咬性昆虫。其可以有效驱赶刺蝇、蠓、黑蝇、恙螨、鹿蝇、跳蚤、蚋、马蝇、蚊子、沙蝇、小飞虫、厩蝇和扁虱等。
避蚊胺于二战期间由美国农业部开发并拥有专利,1946年被指定为美军驱蚊产品。1957年在美国环保局登记注册后开始民用。据统计,每年约有40%的美国人使用含避蚊胺的昆虫驱避剂,全球大约2亿人使用。自1953年以来,已有超过2万个研究单位的研究表明,避蚊胺是市场上最安全、有效的广谱驱避剂。世界卫生组织也推荐使用含有避蚊胺的产品来防止病媒昆虫侵害。
避蚊胺的作用机制避蚊胺的具体作用机制尚不明确。最初认为它通过产生威慑性气味让吸血昆虫产生抗拒,从而避免靠近人体。然而,研究发现避蚊胺能够抑制蚊虫对乳酸、1-辛烯-3-醇等人体汗液主要成分的电生理反应,掩盖或封闭蚊虫嗅觉系统,阻止它们识别合适的猎物。进一步研究表明,避蚊胺直接作用于蚊虫触角上的特殊嗅觉神经元,产生驱避效果,并不抑制其对乳酸、二氧化碳和1-辛烯-3-醇等化学物质的感知。
最新研究还揭示了避蚊胺与某些分子靶标的结合是识别外界物质的第一步生化反应。不过,这些发现仍需进一步验证。
美国摩弗莱公司一步合成法美国摩弗莱公司成立于1927年,位于格林斯博罗市,拥有40多年生产和销售避蚊胺的历史,为全球提供60%的消费量。其用户包括庄臣、拜耳等知名企业。摩弗莱驱蚊霜配方具有化妆品特性,对人体皮肤无刺激,涂抹更均匀且舒适,不易产生干燥紧绷或油腻感。其生产方法称为“一步合成法”,这一过程不会产生化学合成附加物——磷酸、盐酸、盐酸盐和间位异构体混合物。研究表明,雄性大白鼠急性口服LD50值约为2000毫克/公斤,并且含有1%避蚊胺的饲料喂养大鼠200天没有不良作用。
安全性美国疾控中心(CDC)和美国儿科学会(AAP)均推荐使用避蚊胺、派卡瑞丁和驱蚊酯等成分来防止蚊虫叮咬。避蚊胺是目前最有效且安全可靠的驱蚊成分,可放心使用。出于谨慎考虑,2个月以下的婴儿应禁用避蚊胺;3岁以下儿童使用“纯天然植物精华”柠檬桉亦需谨慎。
化学性质避蚊胺为无色至琥珀色液体,具有轻微柑橘香气味,几乎不溶于水但可与乙醇、2-丙醇、丙二醇和棉籽油混溶。工业品间位异构体含量85~95%,d24为0.996~0.998,30℃时粘度为13.3厘泊。雄性大白鼠急性口服LD50值约为2000毫克/公斤;含有1%避蚊胺的饲料喂养大鼠200天没有不良作用;未稀释的化合物能刺激粘膜,但每天使用驱避浓度的避蚊胺涂脸和手臂上只会引起轻微刺激。
用途避蚊胺作为昆虫驱避剂广泛应用于各种固、液体驱蚊系列产品中。三种异构体对蚊虫均有驱避作用,而间位异构体效果最强。其制剂包括70%和95%液体产品。由间甲苯酰氯与二乙胺反应可制备得到。
生产方法上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 2-bromo-N,N-diethyl-5-methylbenzamide —— C12H16BrNO 270.169 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 omega-羟基-避蚊胺 N,N-Diethyl-m-hydroxymethylbenzamide 72236-22-7 C12H17NO2 207.272 N-乙基-3-甲基-苯甲酰胺 N-ethyl-m-Toluamide 26819-07-8 C10H13NO 163.219 避蚊胺omega-羧酸 m-(diethylaminocarbonyl)benzoic acid 72236-23-8 C12H15NO3 221.256 —— N,N-diethyl-4-hydroxy-3-methylphenylamide 108898-76-6 C12H17NO2 207.272 4-氨基-N,N-二乙基-3-甲基苯甲酰胺 4-amino-N,N-diethyl-3-methylbenzamide 926229-45-0 C12H18N2O 206.288 —— N,N-diethyl-2-hydroxy-5-methylbenzamide 474353-19-0 C12H17NO2 207.272 —— N,N-diethyl-2-hydroxy-3-methylphenylamide 85630-34-8 C12H17NO2 207.272 3-(乙基氨基甲酰基)苯甲酸 m-(ethylaminocarbonyl)benzoic acid 126926-33-8 C10H11NO3 193.202 —— N-ethyl-4-hydroxy-3-methylphenylamide —— C10H13NO2 179.219 —— N-ethyl-N-(3-methylbenzyl)ethanamine 22704-60-5 C12H19N 177.29 N,N-二乙基-3-羟基苯甲酰胺 N,N-diethyl-3-hydroxybenzamide 15789-04-5 C11H15NO2 193.246 —— 3-(difluoromethoxy)-N,N-diethyl-5-methylbenzamide 1454765-40-2 C13H17F2NO2 257.28 —— N,N-diethyl-3-methyl-4-nitrobenzamide —— C12H16N2O3 236.271 间甲苯乙酰胺 m-toluamide 618-47-3 C8H9NO 135.166 —— N,N-diethyl-trihydroxy-3-methylphenylamide —— C12H17NO4 239.271 - 1
- 2
反应信息
-
作为反应物:描述:避蚊胺 在 bis(cyclopentadienyl)dihydrozirconium 、 频那醇硼烷 、 盐酸 作用下, 以 乙醚 为溶剂, 20.0 ℃ 、101.33 kPa 条件下, 反应 12.0h, 以60%的产率得到N-ethyl-N-(3-methylbenzyl)ethylamine hydrochloride参考文献:名称:一种酰胺化合物还原制备胺类化合物的方法摘要:本发明涉及一种酰胺化合物还原制备胺类化合物的方法,该方法是指在保护气氛中,将酰胺类化合物或环状酰胺、锆金属催化剂、频哪醇硼烷混合,于室温条件下进行酰胺还原反应,12~48h后通过氯化氢的乙醚溶液后处理,即得胺的盐酸盐化合物。本发明操作简单、成本低且具有良好的官能团耐受性和广泛的底物范围。公开号:CN112299938A
-
作为产物:描述:参考文献:名称:Ortho-metalated aryl tert-butyl sulfones. Comparison with other directing groups and new methodology for polysubstituted aromatics摘要:DOI:10.1021/jo00262a012
-
作为试剂:参考文献:名称:Nanostructured CeO 2 as catalysts for different AOPs based in the application of ozone and simulated solar radiation摘要:DOI:10.1016/j.cattod.2016.04.034
文献信息
-
[EN] BICYCLYL-SUBSTITUTED ISOTHIAZOLINE COMPOUNDS<br/>[FR] COMPOSÉS ISOTHIAZOLINE SUBSTITUÉS PAR UN BICYCLYLE申请人:BASF SE公开号:WO2014206910A1公开(公告)日:2014-12-31The present invention relates to bicyclyl-substituted isothiazoline compounds of formula (I) wherein the variables are as defined in the claims and description. The compounds are useful for combating or controlling invertebrate pests, in particular arthropod pests and nematodes. The invention also relates to a method for controlling invertebrate pests by using these compounds and to plant propagation material and to an agricultural and a veterinary composition comprising said compounds.本发明涉及公式(I)中变量如索权和说明中所定义的自行车基取代异噻唑啉化合物。这些化合物对抗或控制无脊椎动物害虫,特别是节肢动物害虫和线虫方面具有用途。该发明还涉及一种通过使用这些化合物来控制无脊椎动物害虫的方法,以及包含所述化合物的植物繁殖材料、农业和兽医组合物。
-
[EN] AZOLINE COMPOUNDS<br/>[FR] COMPOSÉS AZOLINE申请人:BASF SE公开号:WO2015128358A1公开(公告)日:2015-09-03The present invention relates to azoline compounds of formula (I) wherein A, B1, B2, B3, G1, G2, X1, R1, R3a, R3b, Rg1 and Rg2 are as defined in the claims and the description. The compounds are useful for combating or controlling invertebrate pests, in particular arthropod pests and nematodes. The invention also relates to a method for controlling invertebrate pests by using these compounds and to plant propagation material and to an agricultural and a veterinary composition comprising said compounds.本发明涉及式(I)的噁唑啉化合物,其中A、B1、B2、B3、G1、G2、X1、R1、R3a、R3b、Rg1和Rg2如权利要求和描述中所定义。这些化合物对抗或控制无脊椎动物害虫,特别是节肢动物害虫和线虫方面具有用途。该发明还涉及一种利用这些化合物控制无脊椎动物害虫的方法,以及包括所述化合物的植物繁殖材料、农业和兽医组合物。
-
[EN] MICROBIOCIDAL OXADIAZOLE DERIVATIVES<br/>[FR] DÉRIVÉS D'OXADIAZOLE MICROBIOCIDES申请人:SYNGENTA PARTICIPATIONS AG公开号:WO2017157962A1公开(公告)日:2017-09-21Compounds of the formula (I) wherein the substituents are as defined in claim 1, useful as a pesticides, especially fungicides.式(I)的化合物,其中取代基如权利要求1所定义,作为杀虫剂特别是杀菌剂有用。
-
[EN] INSECTICIDAL TRIAZINONE DERIVATIVES<br/>[FR] DÉRIVÉS DE TRIAZINONE INSECTICIDES申请人:SYNGENTA PARTICIPATIONS AG公开号:WO2013079350A1公开(公告)日:2013-06-06Compounds of the formula (I) or (I'), wherein the substituents are as defined in claim 1, are useful as pesticides.式(I)或(I')的化合物,其中取代基如权利要求1所定义的那样,可用作杀虫剂。
-
Nickel-catalyzed reductive defunctionalization of esters in the absence of an external reductant: activation of C–O bonds作者:Yasuaki Iyori、Kenjiro Takahashi、Ken Yamazaki、Yusuke Ano、Naoto ChataniDOI:10.1039/c9cc07710c日期:——
The nickel-catalyzed reductive cleavage of esters in the absence of an external reductant, which involves the cleavage of an inert acyl C–O bond in
O -alkyl esters is reported.镍催化剂在无外部还原剂的情况下,报告了酯的还原裂解,其中涉及对惰性酰基C-O键进行裂解,这种裂解发生在O-烷基酯中。
表征谱图
-
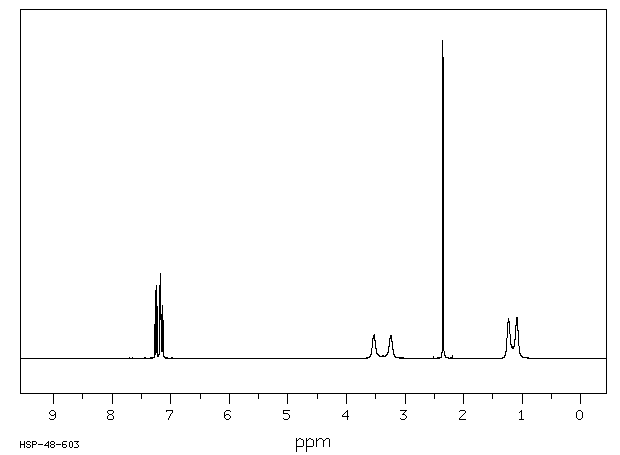
氢谱1HNMR
-
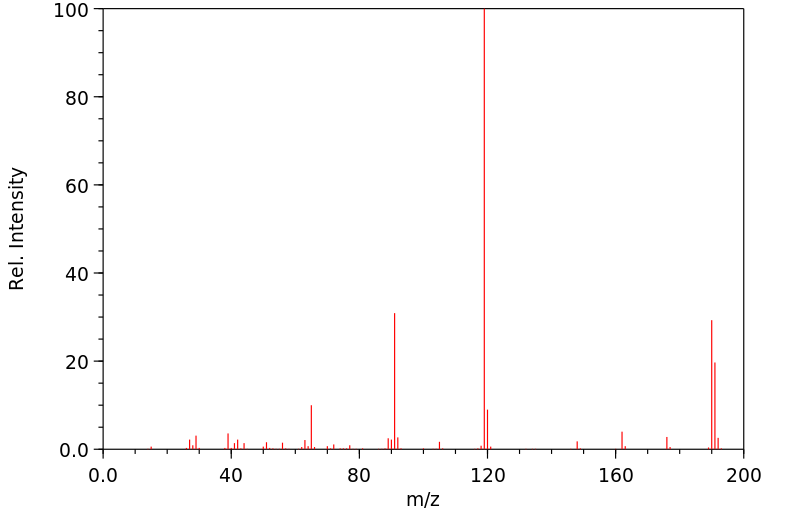
质谱MS
-
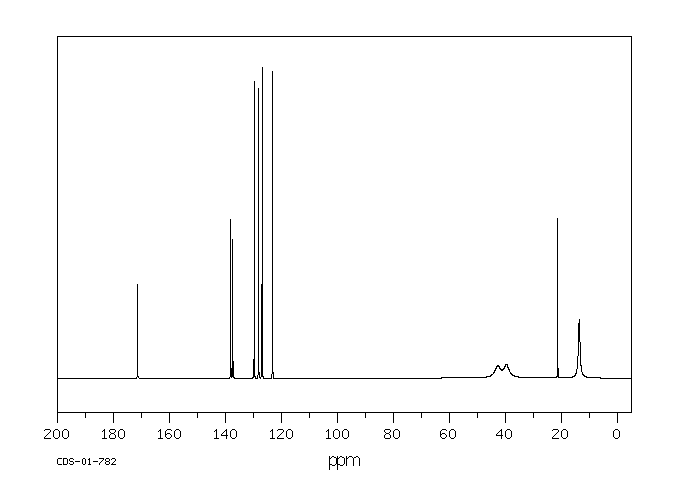
碳谱13CNMR
-
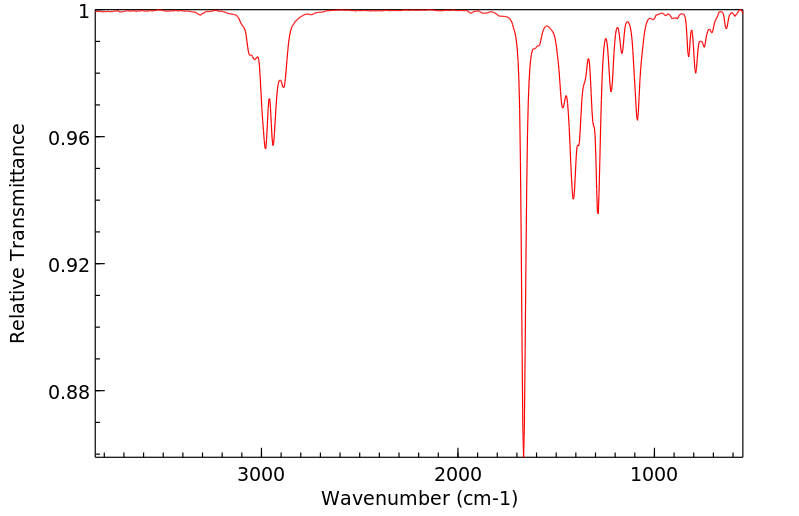
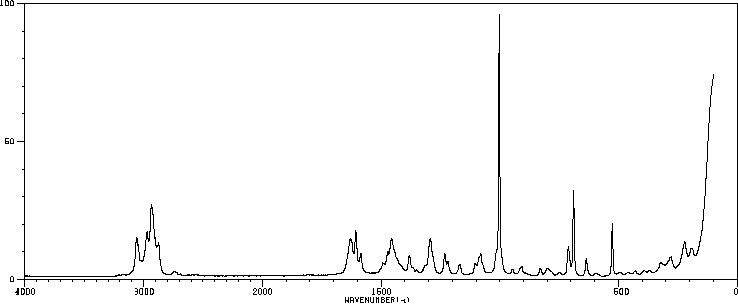
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息