二异丙基氨腈 | 3085-76-5
中文名称
二异丙基氨腈
中文别名
——
英文名称
diisopropylcyanamide
英文别名
N,N-diisopropylcyanamide;di(propan-2-yl)cyanamide
CAS
3085-76-5
化学式
C7H14N2
mdl
MFCD00008865
分子量
126.202
InChiKey
DGCUISYKMONQDH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-27°C(lit.)
-
沸点:93-94 °C25 mm Hg(lit.)
-
密度:0.839 g/mL at 25 °C(lit.)
-
闪点:174 °F
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:9
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.857
-
拓扑面积:27
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险品标志:Xn
-
安全说明:S26,S27,S36/37/39
-
危险类别码:R20/21/22,R36/37/38
-
WGK Germany:3
-
储存条件:| 室温 |
SDS
| Name: | Diisopropylcyanamide 97+% Material Safety Data Sheet |
| Synonym: | None known |
| CAS: | 3085-76-5 |
Synonym:None known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 3085-76-5 | Diisopropylcyanamide | 97+ | 221-401-9 |
Risk Phrases: 20/21
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful by inhalation and in contact with skin.
Potential Health Effects
Eye:
May cause eye irritation. May cause chemical conjunctivitis and corneal damage.
Skin:
May cause irritation and dermatitis. May cause cyanosis of the extremities.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated.
Inhalation:
The toxicological properties of this substance have not been fully investigated. Aspiration may lead to pulmonary edema. Inhalation at high concentrations may cause CNS depression and asphixiation.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Use water spray to keep fire-exposed containers cool. Water may be ineffective.
Material is lighter than water and a fire may be spread by the use of water. Combustible liquid. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas.
Containers may explode when heated.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Remove all sources of ignition. Use a spark-proof tool. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Empty containers retain product residue, (liquid and/or vapor), and can be dangerous. Keep container tightly closed. Keep away from heat, sparks and flame.
Avoid ingestion and inhalation. Do not pressurize, cut, weld, braze, solder, drill, grind, or expose empty containers to heat, sparks or open flames.
Storage:
Keep away from heat, sparks, and flame. Keep away from sources of ignition. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 3085-76-5: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: off-white
Odor: amine-like
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 93 - 94 deg C @ 25.00mm Hg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: 78 deg C ( 172.40 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: .8390g/cm3
Molecular Formula: C7H14N2
Molecular Weight: 126.20
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, ignition sources, excess heat.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 3085-76-5 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Diisopropylcyanamide - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: TOXIC LIQUID, ORGANIC, N.O.S.*
Hazard Class: 6.1
UN Number: 2810
Packing Group: III
IMO
Shipping Name: TOXIC LIQUID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2810
Packing Group: III
RID/ADR
Shipping Name: TOXIC LIQUID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2810
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 20/21 Harmful by inhalation and in contact with
skin.
Safety Phrases:
S 23 Do not inhale gas/fumes/vapour/spray.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 3085-76-5: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 3085-76-5 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 3085-76-5 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:参考文献:名称:Malik, Abdul; Afza, Nighat; Siddiqui, Salimuzzaman, Zeitschrift fur Naturforschung, Teil B: Anorganische Chemie, Organische Chemie, 1982, vol. 37, # 4, p. 512 - 518摘要:DOI:
-
作为产物:参考文献:名称:Jochims, Johannes C.; Abu-El-Halawa, Rajab; Zsolnai, Laszlo, Chemische Berichte, 1984, vol. 117, # 3, p. 1161 - 1177摘要:DOI:
-
作为试剂:参考文献:名称:Polymeric salen-Ti(IV) or V(V) complex catalyzed asymmetric synthesis of O-acetylcyanohydrins from KCN, Ac2O and aldehydes摘要:Polymeric salen-Ti(IV) and V(V) complexes were employed in the enantioselective O-acetyl cyanation of aldehydes with potassium cyanide and acetic anhydride. The crosslinked polymeric salen-Ti(IV) catalyst exhibited good activities and enantioselectivities, up to 91 % ee with 99 % conversion was obtained at -20degreesC with 1 mol % of catalyst (based on bimetallic catalytic unit). Moreover, six consecutive recyclings with the easily recovered crosslinked polymeric catalyst showed no obvious decrease in either activity or enantioselectivity. Linear polymeric salen-V(V) catalyst showed good catalytic efficiency too, up to 94 % ee with 99 % conversion was obtained at -42degreesC with 5 mol % of catalyst. (C) 2004 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tet.2004.08.078
文献信息
-
Ru/C: a simple heterogeneous catalyst for the amination of azoles under ligand free conditions作者:K. Harsha Vardhan Reddy、B. S. P. Anil Kumar、V. Prakash Reddy、R. Uday Kumar、Y. V. D. NageswarDOI:10.1039/c4ra05447d日期:——
A ligand free Ru/C-catalyzed amination of 2-halo azoles with a broad scope of aminating reagents has been developed. Utilizing this protocol a variety of 2-aminoazole derivatives were synthesized in moderate to good yields. The methodology is operationally simple and it provides potentially useful products by using an inexpensive recyclable catalytic system.
-
Formation of Acyl-Substituted Nitrile Ylides by Rh<sub>2</sub>(OAc)<sub>4</sub>-Catalyzed Decomposition of<i>α</i>-Diazocarbonyl Compounds in Nitriles作者:Kazuaki Fukushima、Toshikazu IbataDOI:10.1246/bcsj.68.3469日期:1995.12The Rh2(OAc)4-catalyzed reactions of α-diazocarbonyl compounds in nitrile in the presence of dimethyl acetylenedicarboxylate (DMAD) gave oxazole and pyrrole derivatives. The formation of the oxazole derivatives is explained in terms of the 1,5-cyclization of an acyl-substituted nitrile ylide intermediate, and the formation of the pyrrole derivatives is explained by the 1,3-dipolar cycloaddition of
-
Synthesis of Cyanamides from Cyanogen Bromide under Mild Conditions through N-Cyanation of Allylic Tertiary Amines作者:Chenghui Sun、Honggang Liang、Lingxiang Bao、Yao Du、Yiying Zhang、Siping PangDOI:10.1055/s-0036-1588533日期:2017.12Cyanamides were selectively formed through a one-step nucleophilic substitution reaction of allylic tertiary amines with cyanogen bromide. Because of the mild reaction conditions and good yields of the reaction, as well as the commercial availability of the starting materials, this new method represents a valuable tool for the synthesis of cyanamides through an N-deallylation reaction and an N-cyanation
-
Prototropy in the System -ClCH-N=N-; Cycloadditions of 1-Aza-2-azoniaallene Cations Derived from Aldehydes作者:Yiping Guo、Quanrui Wang、Johannes C. JochimsDOI:10.1055/s-1996-4189日期:1996.21,3-Disubstituted 1-aza-2-azoniaallene salts 10 were obtained as reactive intermediates on oxidation of aldehyde hydrazones 7 with tert-butyl hypochlorite and treatment of the resulting (1-chloroalkyl)azo compounds 8 with a Lewis acid (SbCl5). The allenes 10 underwent cycloadditions to the multiple bonds of nitriles, acetylenes, and olefins affording the heterocyclic salts 12, which gave the free bases 12’ with aqueous NaOH. The critical point of this sequence lies in the prototropic rearrangement of 8 into the hydrazonyl chlorides 9, from which no heterocumulenes 10 could be obtained. However, further chlorination of 7c led to the (1,1-dichloropropyl)azo compound 13, which gave the chloro substituted allenium salt 14 on treatment with SbCl5. Cycloaddition of 14 to acetonitrile afforded the chloro substituted 1,2,4-triazolium salt 16. Conditions for slow rearrangements 8 → 9 were found for compounds 8 derived from primary or secondary alkanecarbaldehydes. However, for (1-chloroalkyl)azo compounds derived from benzaldehyde or pivaldehyde the prototropy 8 → 9 was too fast to permit preparation of heterocumulenes 10.1,3-二取代的1-氮-2-氮杂烯盐10作为活性中间体,通过醛肼7在叔丁基次氯酸酯氧化下制得,并对生成的(1-氯烷基)偶氮化合物8用路易斯酸(SbCl5)处理.烯盐10经与腈、炔烃、或烯烃的复分解反应得到含氮盐12,用NaOH水溶液处理即得到自由碱12'.本反应历程的关键点在于8向肼叉基氯化物9的质子迁移,而从9不能得到含异氰化合物10.而7c的进一步氯化导致(1,1-二氯丙基)偶氮化合物13生成,用SbCl5处理得到氯取代的烯盐14,与乙腈的复分解反应得到氯取代的1,2,4-三氮唑盐16.在由伯或仲脂肪醛衍生的(1-氯烷基)偶氮化合物8中,发现了缓慢的8-9重排反应,但对于由苯甲醛或新戊醛衍生的(1-氯烷基)偶氮化合物8而言,8-9重排过快,无法制得含异氰化合物10.
-
一种由烯丙基叔胺类化合物一步合成氰基叔 胺类化合物的方法
表征谱图
-
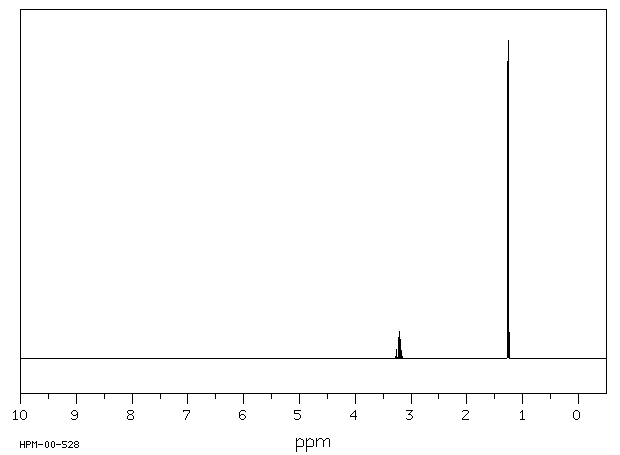
氢谱1HNMR
-
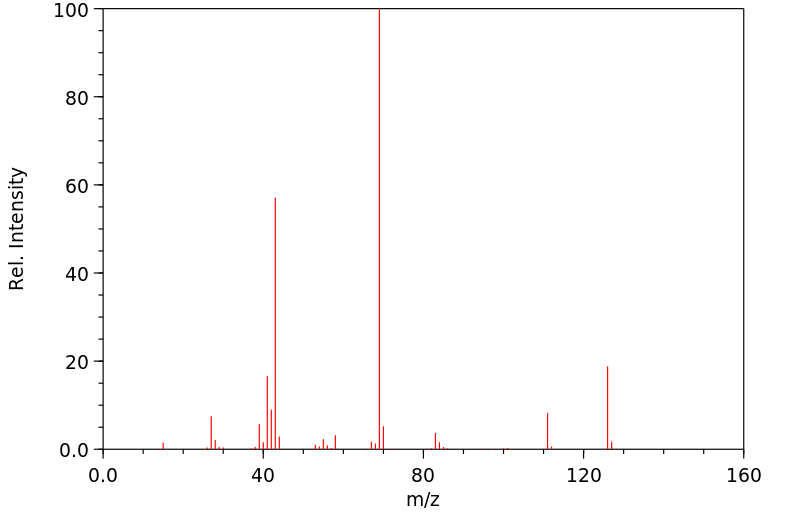
质谱MS
-
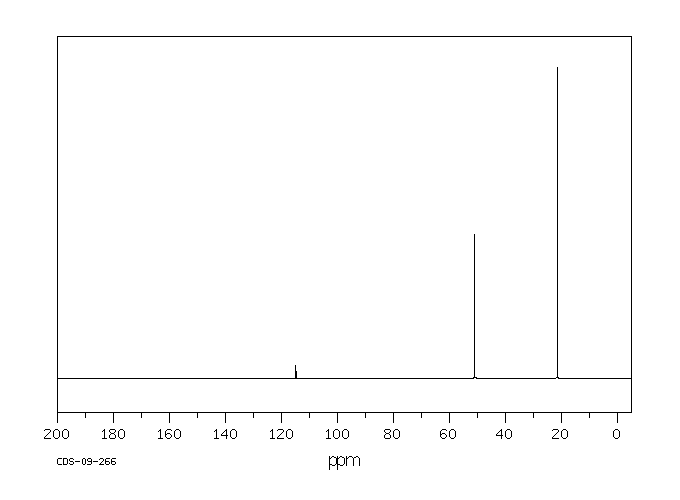
碳谱13CNMR
-
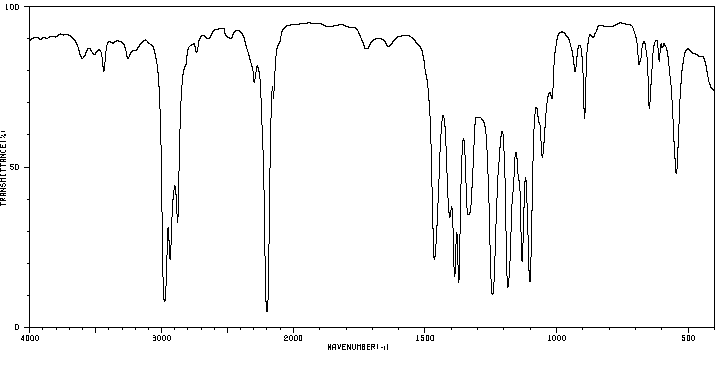
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷