N-甲基-N-(亚甲基氨基)甲胺 | 2035-89-4
中文名称
N-甲基-N-(亚甲基氨基)甲胺
中文别名
甲醛二甲基腙
英文名称
N-methyl-N-(methyleneamino)methanamine
英文别名
formaldehyde dimethylhydrazone;formaldehyde N,N-dimethylhydrazone;1,1-dimethyl-2-methylenehydrazine;FDMH;Dimethylmethylenehydrazine;N-methyl-N-(methylideneamino)methanamine
CAS
2035-89-4
化学式
C3H8N2
mdl
——
分子量
72.1099
InChiKey
NDNVSJIXYFNRDR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-103°C(lit.)
-
沸点:71°C(lit.)
-
密度:0.8042 (rough estimate)
-
最大波长(λmax):242nm(Heptane)(lit.)
-
保留指数:593;593
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:5
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:0.67
-
拓扑面积:15.6
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险等级:3
-
海关编码:2928000090
-
储存条件:存储条件:0-10°C,需置于惰性气体环境中,避免与空气接触和受热。
SDS
甲醛二甲基腙 修改号码:5.3
模块 1. 化学品
产品名称: Formaldehyde Dimethylhydrazone
修改号码: 5.3
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第2级
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 高度易燃液体和蒸气
防范说明
[预防] 远离热源/火花/明火/热表面。禁烟。
保持容器密闭。
使用防爆的电气/通风/照明设备。采取预防措施以防静电和火花引起的着火。
穿戴防护手套/护目镜/防护面具。
[急救措施] 皮肤接触:立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
[储存] 存放于通风良好处。保持凉爽。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 甲醛二甲基腙
百分比: >98.0%(GC)(T)
CAS编码: 2035-89-4
分子式: C3H8N2
甲醛二甲基腙 修改号码:5.3
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:喷水,保持容器冷却。如果安全,消除一切火源。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 回收到密闭容器前用干砂或惰性吸收剂吸收泄漏物。一旦大量泄漏,筑堤控制。附着
物或收集物应该根据相关法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离热源/火花/明火
/热表面。禁烟。采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 如果可能,使用封闭系统。如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于冷藏防爆冰箱。
存放于惰性气体环境中。
远离不相容的材料比如氧化剂存放。
热敏, 气敏
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
甲醛二甲基腙 修改号码:5.3
模块 9. 理化特性
颜色: 无色-微浅黄色
气味: 无资料
pH: 无数据资料
熔点:
-103°C
沸点/沸程 71 °C
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.81
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 火花, 明火, 静电
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: ipr-mus LD50:239 mg/kg
ivn-rat LDLo:100 mg/kg
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
RTECS 号码: LP9000000
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧,焚烧时需要特别注
意该物质是高度可燃的。废弃处置时请遵守国家、地区和当地的所有法规。
甲醛二甲基腙 修改号码:5.3
模块 14. 运输信息
联合国分类: 第3类 易燃液体 。
UN编号: 1993
正式运输名称: 易燃液体, 不另作详细说明
包装等级: II
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: Formaldehyde Dimethylhydrazone
修改号码: 5.3
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第2级
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 高度易燃液体和蒸气
防范说明
[预防] 远离热源/火花/明火/热表面。禁烟。
保持容器密闭。
使用防爆的电气/通风/照明设备。采取预防措施以防静电和火花引起的着火。
穿戴防护手套/护目镜/防护面具。
[急救措施] 皮肤接触:立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
[储存] 存放于通风良好处。保持凉爽。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 甲醛二甲基腙
百分比: >98.0%(GC)(T)
CAS编码: 2035-89-4
分子式: C3H8N2
甲醛二甲基腙 修改号码:5.3
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:喷水,保持容器冷却。如果安全,消除一切火源。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 回收到密闭容器前用干砂或惰性吸收剂吸收泄漏物。一旦大量泄漏,筑堤控制。附着
物或收集物应该根据相关法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离热源/火花/明火
/热表面。禁烟。采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 如果可能,使用封闭系统。如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于冷藏防爆冰箱。
存放于惰性气体环境中。
远离不相容的材料比如氧化剂存放。
热敏, 气敏
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
甲醛二甲基腙 修改号码:5.3
模块 9. 理化特性
颜色: 无色-微浅黄色
气味: 无资料
pH: 无数据资料
熔点:
-103°C
沸点/沸程 71 °C
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.81
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 火花, 明火, 静电
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: ipr-mus LD50:239 mg/kg
ivn-rat LDLo:100 mg/kg
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
RTECS 号码: LP9000000
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧,焚烧时需要特别注
意该物质是高度可燃的。废弃处置时请遵守国家、地区和当地的所有法规。
甲醛二甲基腙 修改号码:5.3
模块 14. 运输信息
联合国分类: 第3类 易燃液体 。
UN编号: 1993
正式运输名称: 易燃液体, 不另作详细说明
包装等级: II
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 偏二甲肼 1,1-dimethylhydrazine 57-14-7 C2H8N2 60.0989
反应信息
-
作为反应物:描述:参考文献:名称:Zhigach,A.F. et al., Journal of general chemistry of the USSR, 1973, vol. 43, p. 1946 - 1948摘要:DOI:
-
作为产物:描述:dimethylhydrazine 在 氯胺 作用下, 生成 N-甲基-N-(亚甲基氨基)甲胺参考文献:名称:Delalu, Henri; Marchand, Alain, Journal de Chimie Physique et de Physico-Chimie Biologique, 1987, vol. 84, # 7-8, p. 997 - 1002摘要:DOI:
文献信息
-
Ammonia–dimethylchloramine system: Kinetic approach in an aqueous medium and comparison with the mechanism involving liquid ammonia作者:J. Stephan、V. Pasquet、M. Elkhatib、V. Goutelle、H. DelaluDOI:10.1002/kin.20312日期:2008.6medium. Dimethylchloramine prepared in a pure state undergoes dehydrohalogenation in an alkaline medium: the principal products formed are N-methylmethanimine, 1,3,5-trimethylhexahydrotriazine, formaldehyde, and methylamine. The kinetics of this reaction was studied by UV, GC, and HPLC as a function of temperature, initial concentrations of sodium hydroxide, and chlorinated derivative. The reaction is of在对液氨中的氨-二甲基氯胺系统进行了详尽的研究之后,比较该系统在液氨中的反应性与相同系统在水性介质中的反应性是很有趣的。以纯态制备的二甲基氯胺在碱性介质中进行脱卤化氢:形成的主要产物是 N-甲基甲亚胺、1,3,5-三甲基六氢三嗪、甲醛和甲胺。该反应的动力学通过 UV、GC 和 HPLC 作为温度、氢氧化钠初始浓度和氯化衍生物的函数进行了研究。该反应是二级反应,遵循 E2 机理(k1 = 4.2 × 10-5 M-1 s-1,ΔH○# = 82 kJ mol-1,ΔS○# = -59 J mol-1 K-1 )。二甲基氯胺氧化不对称二甲基肼涉及两个连续的过程。第一步遵循关于卤胺和肼的一级定律,导致形成氨基氮烯中间体 (k2 = 150 × 10-5 M-1 s-1)。第二步对应于在 pH 13) 下将氨基氮烯转化为甲醛二甲腙。该反应遵循一阶定律 (k3 = 23.5 × 10-5 s-1)。二甲基氯胺-氨相互作用对应于
-
A Novel, Simple Cyclocondensation Reaction Towards Glycosyl Triazines作者:David Deniaud、Vincent Kikelj、Karine Julienne、Pascal Janvier、Jean-Claude MeslinDOI:10.1055/s-0028-1083156日期:——Sugars bearing an isothiocyanate moiety at C-1 react with diazadienium iodide to afford glycosyl triazines that represent, through an easy cyclocondensation reaction step, a flexible entry to different nucleoside analogues. We herein demonstrate that this [4+2] cycloaddition reaction occurs with total regiocontrol and good yields. Subsequent transformation of the thiocarbonyl into a carbonyl, and nucleophilic substitution of the methylsulfanyl group by ammonia, yields the 5-azacytidine analogues. All compounds were fully characterised by IR, HRMS, and ¹³C and ¹H NMR (COSY, HMBC and HMQC).
-
<i>N</i> , <i>N</i> ‐Dialkylhydrazones as Versatile Umpolung Reagents in Enantioselective Anion‐Binding Catalysis作者:Melania Gómez‐Martínez、María Carmen Pérez‐Aguilar、Dariusz G. Piekarski、Constantin G. Daniliuc、Olga García MancheñoDOI:10.1002/anie.202013380日期:2021.3ion‐pair complex was envisioned. The formation of such a network was further supported by both experimental and computational studies, which showed the crucial role of the anion as a template unit. The asymmetric Reissert‐type reaction of quinolines as a model test reaction chemoselectively delivered highly enantiomerically enriched hydrazones (up 95:5 e.r.) that could be further derivatized to value‐added
-
Stereoselective Nucleophilic Formylation and Cyanation of α-Alkoxy- and α-Aminoaldehydes作者:Rosario Fernández、Eloísa Martín-Zamora、Carmen Pareja、José M. LassalettaDOI:10.1021/jo015711+日期:2001.7.1formaldehyde N,N-dialkylhydrazones to carbohydrate-derived alpha-alkoxyaldehydes takes place under neutral conditions and in the absence of catalysts or promoters to afford the corresponding alpha-hydroxyhydrazones in good to excellent yields and with highly anti diastereoselectivities. Subsequent transformations of the hydrazono group into aldehydes and nitriles following known procedures provide a new entry
-
Divergent Synthesis and Real-Time Biological Annotation of Optically Active Tetrahydrocyclopenta[<i>c</i>]pyranone Derivatives作者:Shawn D. Nelson、Mathias J. Wawer、Stuart L. SchreiberDOI:10.1021/acs.orglett.6b03118日期:2016.12.16molecular shapes beyond planar geometries. With this in mind, a collection of single-enantiomer bicyclic, fused cyclopentenones underpinned by a complexity-generating Pauson–Khand cyclization was synthesized. A fingerprint of biological actions of these compounds was determined immediately after synthesis using real-time annotation−a process relying on multiplexed measurements of alterations in cell morphological
表征谱图
-
氢谱1HNMR
-
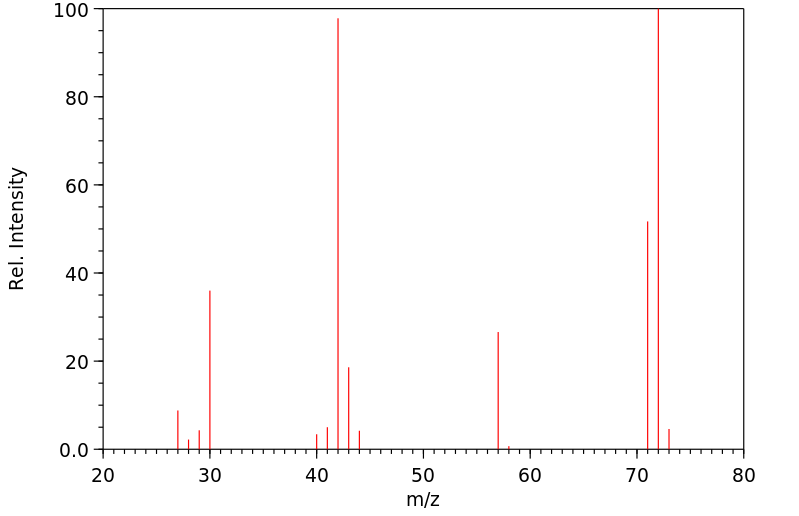
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷