丁酮肟 | 96-29-7
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-30 °C
-
沸点:59-60 °C15 mm Hg(lit.)
-
密度:0.924 g/mL at 25 °C(lit.)
-
蒸气密度:3 (vs air)
-
闪点:140 °F
-
溶解度:水: 25°C时可溶100g/L
-
介电常数:3.4(20℃)
-
LogP:0.63 at 25℃
-
物理描述:Methyl ethyl ketoxime is a clear colorless liquid with a musty odor. (NTP, 1992)
-
颜色/状态:Liquid
-
蒸汽密度:3 (NTP, 1992) (Relative to Air)
-
蒸汽压力:1.06 mm Hg at 20 °C
-
分解:When heated to decomposition it emits toxic fumes of /oxides of nitrogen/.
-
折光率:Index of refraction = 1.4410 at 20 °C/D
-
解离常数:pKa = 12.45 at 25 °C
-
保留指数:793
-
稳定性/保质期:
避免与酸或氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):0.7
-
重原子数:6
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:32.6
-
氢给体数:1
-
氢受体数:2
ADMET
安全信息
-
TSCA:Yes
-
危险等级:3
-
危险品标志:Xn
-
安全说明:S13,S23,S25,S26,S36/37,S36/37/39,S45,S61
-
危险类别码:R40,R41,R21,R43
-
WGK Germany:1
-
海关编码:29280090
-
危险品运输编号:UN 1993 3/PG 3
-
RTECS号:EL9275000
-
包装等级:III
-
危险类别:3
-
储存条件:1. 储存在阴凉、通风的仓库中,并远离火源和热源,确保容器密封。与氧化剂分开存放,禁止混存。 2. 配备适当的消防器材,并在储区准备泄漏应急处理设备及合适的吸收材料。
SDS
| Name: | 2-Butanone oxime 99% Material Safety Data Sheet |
| Synonym: | Ethyl methyl ketone oxime; Ethyl methyl ketoxime; 2-Butanone oxime; Methyl ethyl ketoxime; MEK-oxime |
| CAS: | 96-29-7 |
Synonym:Ethyl methyl ketone oxime; Ethyl methyl ketoxime; 2-Butanone oxime; Methyl ethyl ketoxime; MEK-oxime
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 96-29-7 | Methyl ethyl ketoxime | 99 | 202-496-6 |
Risk Phrases: 21 40 41 43
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful in contact with skin. Limited evidence of a carcinogenic effect. Risk of serious damage to eyes. May cause sensitization by skin contact.
Potential Health Effects
Eye:
Causes severe eye irritation.
Skin:
Harmful if absorbed through the skin. May cause skin sensitization, an allergic reaction, which becomes evident upon re-exposure to this material.
Ingestion:
Methemoglobinemia is characterized by dizziness, drowsiness, headache, shortness of breath, cyanosis (bluish discoloration of skin due to deficient oxygenation of the blood), rapid heart rate and chocolate-brown colored blood. May be harmful if swallowed.
Overexposure may cause methemoglobinemia.
Inhalation:
Not available.
Chronic:
Chronic exposure may cause liver damage.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Remove contaminated clothing and shoes.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Get medical aid immediately.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Flammable liquid and vapor. Vapors are heavier than air and may travel to a source of ignition and flash back. Vapors can spread along the ground and collect in low or confined areas.
Extinguishing Media:
Use extinguishing media most appropriate for the surrounding fire.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Clean up spills immediately, observing precautions in the Protective Equipment section. Remove all sources of ignition. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation. Use only with adequate ventilation. Keep away from heat, sparks and flame.
Storage:
Keep away from sources of ignition. Keep container closed when not in use. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use process enclosure, local exhaust ventilation, or other engineering controls to control airborne levels.
Exposure Limits CAS# 96-29-7: Personal Protective Equipment Eyes: Wear chemical splash goggles.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Clear liquid
Color: clear colorless to pale yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: 0.904 mm Hg @ 25 deg C
Viscosity: Not available.
Boiling Point: 152.5 deg C @ 760 mm Hg
Freezing/Melting Point: -29.5 deg C
Autoignition Temperature: Not available.
Flash Point: 59 deg C ( 138.20 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: Slightly soluble.
Specific Gravity/Density: .9200 g/cm3
Molecular Formula: C4H9NO
Molecular Weight: 87.12
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
High temperatures, ignition sources.
Incompatibilities with Other Materials:
Strong oxidizing agents, strong acids.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 96-29-7: EL9275000 LD50/LC50:
CAS# 96-29-7: Draize test, rabbit, eye: 100 uL Severe; Oral, mouse: LD50 = 1 gm/kg; Oral, rat: LD50 = 930 mg/kg; Skin, rabbit: LD50 = 200 uL/kg.
Skin, rabbit: LD50 = 200 uL/kg = 184 mg/kg.
Carcinogenicity:
Methyl ethyl ketoxime - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: FLAMMABLE LIQUIDS, N.O.S.
Hazard Class: 3
UN Number: 1993
Packing Group: III
IMO
Shipping Name: FLAMMABLE LIQUIDS, N.O.S.
Hazard Class: 3
UN Number: 1993
Packing Group: III
RID/ADR
Shipping Name: FLAMMABLE LIQUIDS, N.O.S.
Hazard Class: 3
UN Number: 1993
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 21 Harmful in contact with skin.
R 40 Limited evidence of a carcinogenic effect.
R 41 Risk of serious damage to eyes.
R 43 May cause sensitization by skin contact.
Safety Phrases:
S 13 Keep away from food, drink and animal feeding
stuffs.
S 23 Do not inhale gas/fumes/vapour/spray.
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 36/37/39 Wear suitable protective clothing, gloves
and eye/face protection.
WGK (Water Danger/Protection)
CAS# 96-29-7: 1
Canada
CAS# 96-29-7 is listed on Canada's DSL List.
CAS# 96-29-7 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 96-29-7 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
肟类除氧剂
肟类化合物(如二甲基酮肟、甲乙酮肟、乙醛肟)作为新型除氧剂,于1984年由美国Drew化学公司首次公开专利。这类化合物低毒、高效且速度快,并具有钝化保护作用,在欧美日等发达国家得到广泛应用。我国也在九十年代成功开发并推广了这一技术。
性能与应用 1. 除氧性能肟类化合物是有机含肟基化合物,主要应用于锅炉除氧和停炉保护。常用的肟类化合物包括乙醛肟、二甲基酮肟(丙酮肟)及甲乙酮肟。这类化合物具有较强的还原性,易于与氧气反应。在较宽的温度(138~336℃)和压力范围(0.3~13.7Mpa)内表现出良好的除氧性能。实验表明,在相同条件下,肟类化合物的除氧速度及效率均优于联氨。
2. 缓蚀与钝化作用肟类化合物能够将高价铁、铜氧化物还原为低价态,并在钢材表面形成良好的磁性氧化膜,从而提供优异的钝化和缓蚀效果。其中,二甲基酮肟的效果最佳且用量最少。实验数据显示,肟类化合物的钝化及缓蚀作用与联氨相似,在高温高压条件下,能显著减少溶液中铁含量并对钢材起到保护作用。
3. 挥发性肟类化合物的挥发性高于联氨、DEHA、吗啉和环己胺等物质,接近于NH3。这类除氧剂在蒸汽凝结时,会有一部分药剂溶入凝结水中,有助于保护金属材料不受损害。
4. 分解性高温高压条件下,肟类化合物分解生成NH3、N2、H2O及微量乙酸,并无甲酸产生,对水汽系统没有不良影响。
5. 低毒性根据LD50的数据比较,联氨的毒性最强(290mg/kg),而肟类化合物如乙醛肟(1900mg/kg)、甲乙酮肟(2800mg/kg)和二甲基酮肟(5500mg/kg)的毒性较低。皮肤接触实验表明,肟类除氧剂无明显刺激作用,联氨则可能导致皮肤红肿、粘膜充血等损伤。
化学性质肟类化合物为无色油状液体,熔点-29.5℃,沸点152-153℃(2kPa),相对密度0.9232(20/4℃),折光率1.4410。能与醇、醚混溶,并可溶解于水中的10份水中。
用途主要用于醇酸树脂涂料防结皮剂和硅固化剂,作为防止结皮的抗氧剂使用效果更佳。此外,也用于有机合成及各种油基漆、醇酸漆、环氧酯漆等储运过程中的防结皮处理,并可用作硅固化剂。
生产方法上下游信息
反应信息
-
作为反应物:参考文献:名称:氰尿酰氯和二甲基亚砜在温和条件下促进酮肟的贝克曼重排摘要:酰胺的合成通过在温和条件下DMSO已经报道/由氰尿酰氯(TCT)促进酮肟的贝克曼重排。使用二苯甲酮肟作为底物,研究贝克曼重排的条件,例如溶剂,TCT / DMSO的比例和温度。采用优化的条件得到十四种酰胺,收率范围为20%至99%。根据质谱分析,提出了一种涉及活性二甲基烷氧基s中间体的合理机理。据我们所知,这是第一个研究TCT / DMSO在温和条件下促进酮肟的贝克曼重排以有效提供酰胺的案例。DOI:10.1016/j.tetlet.2020.152707
-
作为产物:参考文献:名称:Lewis acidic strength controlled highly selective synthesis of oxime via liquid-phase ammoximation over titanosilicates摘要:钛硅酸盐的Lewis酸性通过影响Ti-O-OH的反应活化能,决定了氨氧化中肟的选择性,从而高效形成NH2OH。DOI:10.1039/c4ra08799b
-
作为试剂:描述:sodium monochloroacetic acid 在 丁酮肟 、 水 、 sodium hydroxide 作用下, 以 丙酮 为溶剂, 反应 2.0h, 以55%的产率得到羧甲基羟胺半盐酸盐参考文献:名称:α−(アミノオキシ)カルボン酸類の製造方法摘要:题目:提供一种高效的制备α-(烷基亚氨基氧)羧酸类化合物作为醛捕捉剂的原料的α-(烷基亚基氨基氧)羧酸类化合物的方法。 解决方法:通过在酮类溶剂中,在碱的存在下,使式(1)所示的肟和式(2)所示的α-卤代羧酸反应,制备式(3)所示的α-(烷基亚基氨基氧)羧酸或其碱金属盐。(其中,R1,R2是氢原子或烷基,R3是烷基或苯基,X表示卤素原子。)【选项图】无。公开号:JP2020158452A
文献信息
-
Efficient Reductive Deoximation by Tungsten(VI) Chloride (WCl<sub>6</sub>) or Molybdenum(V) Chloride (MoCl<sub>5</sub>) in the Presence of Zn Powder in CH<sub>3</sub>CN作者:Habib Firouzabadi、Arezu Jamalian、Babak KarimiDOI:10.1246/bcsj.75.1761日期:2002.8Tungsten(VI) chloride (WCl6) or molybdenum(V) chloride (MoCl5) in the presence of zinc powder in CH3CN provides an efficient and facile procedure for the deprotection of oximes to their corresponding aldehydes and ketones in high yields.
-
<i>m</i>-CPBA Mediated Metal Free, Rapid Oxidation of Aliphatic Amines to Oximes作者:Vilas V. Patil、Eknath M. Gayakwad、Ganapati S. ShankarlingDOI:10.1021/acs.joc.5b01740日期:2016.2.5An efficient, rapid oxidation of various aliphatic amines to oximes using m-CPBA as an oxidant in ethyl acetate is described. High conversion (100%) with >90% oxime selectivity is achieved at room temperature under catalyst-free conditions. Mild reaction conditions along with an easy work up procedure offer lower byproduct formation and high selectivity for oximes in good yield and purity.
-
一种肟氧化制备硝基烷烃的绿色合成方法
-
Deoximation of Oximes with 2-Iodylbenzoic Acid in Water in the Presence of β-Cyclodextrin作者:K. Rama Rao、N. Srilakshmi Krishnaveni、K. Surendra、Y. V. NageswarDOI:10.1055/s-2003-41448日期:——Oximes of various aldehydes and ketones can be converted to the corresponding carbonyl compounds at room temperature in impressive yields with 2-iodylbenzoic acid in water in the presence of β-cyclodextrin.
-
Brønsted acid catalyzed transoximation reaction: synthesis of aldoximes and ketoximes without use of hydroxylamine salts作者:Kengo Hyodo、Kosuke Togashi、Naoki Oishi、Genna Hasegawa、Kingo UchidaDOI:10.1039/c6gc02156e日期:——The transoximation reaction enables the transfer of oxime to a carbonyl compound and is catalyzed by transoximase in the pupae of silkworm. Inspired by this bio-synthetic pathway, we achieved the...
表征谱图
-
氢谱1HNMR
-
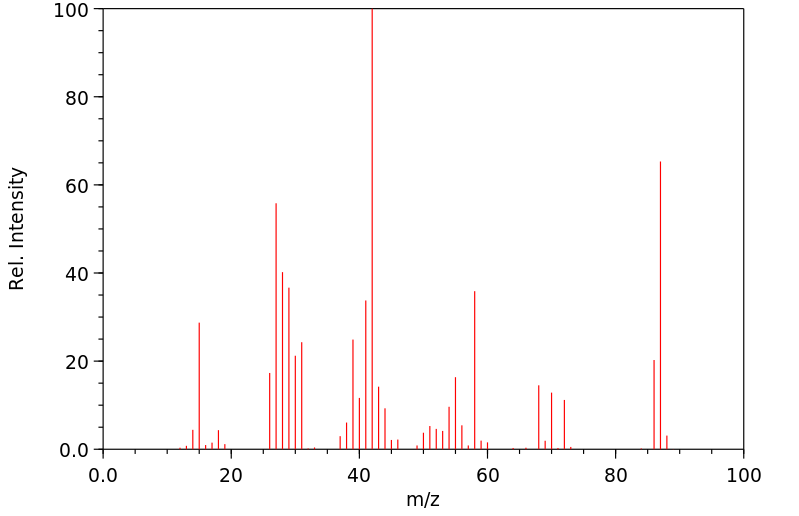
质谱MS
-
碳谱13CNMR
-
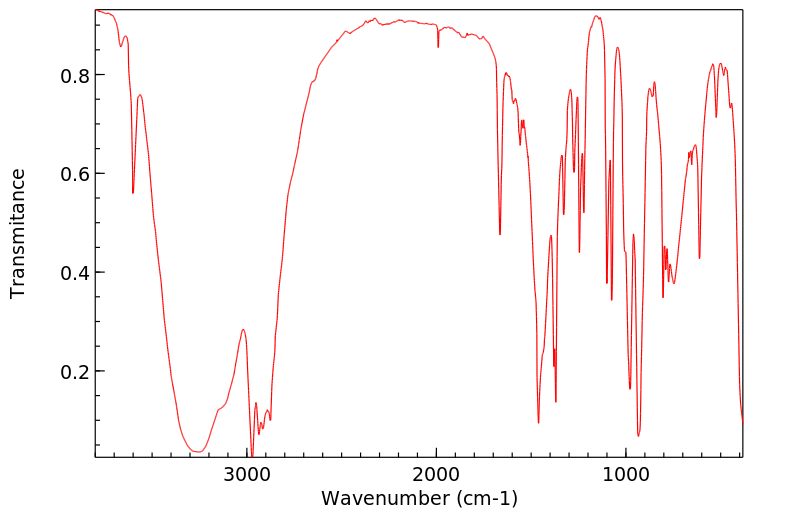
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息