三叔丁基硼酸酯 | 7397-43-5
分子结构分类
中文名称
三叔丁基硼酸酯
中文别名
三叔丁基硼酸
英文名称
tri-(tert-butyl)borate
英文别名
tri-t-butyl borate;Borsaeure-tri-tert-butylester;Tri-tert.-butyl-borat;Tri-tert-butyl borate;tritert-butyl borate
CAS
7397-43-5
化学式
C12H27BO3
mdl
MFCD00009652
分子量
230.156
InChiKey
ZMCWFMOZBTXGKI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:18-19 °C (lit.)
-
沸点:101 °C/74 mmHg (lit.)
-
密度:0.811 g/mL at 25 °C (lit.)
-
闪点:85 °F
-
稳定性/保质期:
在常温常压下稳定,应避免与强氧化剂接触。
计算性质
-
辛醇/水分配系数(LogP):4.18
-
重原子数:16
-
可旋转键数:6
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:27.7
-
氢给体数:0
-
氢受体数:3
安全信息
-
TSCA:No
-
危险等级:3.2
-
安全说明:S16,S33
-
危险类别码:R10
-
海关编码:2920909090
-
包装等级:III
-
危险类别:3.2
-
危险品运输编号:UN 1993 3
-
储存条件:密封储存于阴凉、干燥的库房中,远离火源和易燃易爆区域,并常使用氮气进行保护。
SDS
| Name: | Tri-tert-butyl borate 98% Material Safety Data Sheet |
| Synonym: | Tri-tert-butoxyboran |
| CAS: | 7397-43-5 |
Synonym:Tri-tert-butoxyboran
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 7397-43-5 | Tri-tert-butyl borate | 98% | unlisted |
Risk Phrases: 10
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Flammable.The toxicological properties of this material have not been fully investigated.Moisture sensitive.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May cause gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated. May be harmful if swallowed.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. May be harmful if inhaled.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Do not induce vomiting. If victim is conscious and alert, give 2-4 cupfuls of milk or water. Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Vapors can travel to a source of ignition and flash back. Can burn in a fire, releasing toxic vapors. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Flammable liquid and vapor.
Extinguishing Media:
In case of fire, use water, dry chemical, chemical foam, or alcohol-resistant foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Remove all sources of ignition. Use a spark-proof tool. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Ground and bond containers when transferring material. Use spark-proof tools and explosion proof equipment. Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes. Empty containers retain product residue, (liquid and/or vapor), and can be dangerous. Container should be opened by a technically qualified person. Use only in a chemical fume hood. Do not pressurize, cut, weld, braze, solder, drill, grind, or expose empty containers to heat, sparks or open flames. Keep away from heat, sparks and flame.
Storage:
Keep away from sources of ignition. Store in a cool, dry place.
Store in a tightly closed container. Keep under a nitrogen blanket.
Flammables-area.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate general or local explosion-proof ventilation to keep airborne levels to acceptable levels.
Exposure Limits CAS# 7397-43-5: Personal Protective Equipment Eyes: Wear chemical splash goggles.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear a chemical apron.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: clear, colorless
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 101 deg C @ 74.00mm Hg
Freezing/Melting Point: 18 - 19 deg C
Autoignition Temperature: Not available.
Flash Point: 29 deg C ( 84.20 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: .8110g/cm3
Molecular Formula: C12H27BO3
Molecular Weight: 230.15
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
May decompose on exposure to moist air or water. Moisture sensitive.
Conditions to Avoid:
Ignition sources, excess heat, exposure to moist air or water.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide, oxides of boron.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 7397-43-5 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Tri-tert-butyl borate - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: FLAMMABLE LIQUID, N.O.S.*
Hazard Class: 3
UN Number: 1993
Packing Group: III
IMO
Shipping Name: FLAMMABLE LIQUID, N.O.S.
Hazard Class: 3.3
UN Number: 1993
Packing Group: III
RID/ADR
Shipping Name: FLAMMABLE LIQUID, N.O.S.
Hazard Class: 3
UN Number: 1993
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
R 10 Flammable.
Safety Phrases:
S 16 Keep away from sources of ignition - No
smoking.
WGK (Water Danger/Protection)
CAS# 7397-43-5: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 7397-43-5 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 7397-43-5 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:参考文献:名称:Dicko, Amadou; Coste, Camille; Bastide, Jean, Bulletin de la Societe Chimique de France, 1982, vol. II, p. 153 - 158摘要:DOI:
-
作为产物:参考文献:名称:135.醇-三氯化硼-吡啶体系中的反应顺序摘要:DOI:10.1039/jr9530000667
-
作为试剂:参考文献:名称:[EN] METHOD FOR PRODUCING AN OPTICALLY ACTIVE TETRAHYDROQUINOLINE
[FR] PROCEDE DE PRODUCTION D'UNE TETRAHYDROQUINOLINE ACTIVE SUR LE PLAN OPTIQUE摘要:公开号:WO2004074255A3
文献信息
-
Boric Ester and Thiourea as Coupling Partners in a Copper-Mediated Oxidative Dehydrosulfurative Carbon–Oxygen Cross-Coupling Reaction作者:Hyeji Kim、Jihong Lee、Hyunik Shin、Jeong-Hun SohnDOI:10.1021/acs.orglett.8b00502日期:2018.4.6dehydrosulfurative carbon–oxygen cross-coupling reaction with boric ester and six-membered cyclic thiourea for single-step production of densely substituted 2-alkoxypyrimidines incorporated in a privileged scaffold is described. This is the first demonstration of boric ester acting as an alkoxy donor in a metal-catalyzed coupling reaction to produce ether. The reaction method offers a shortcut for producing
-
Metal Tetrahydroborates and Tetrahydroborato Metallates, 30 [1]. Alkoxo-Substituted Alkali Metal Tetrahydroborates: Studies in Solution and Structures in the Solid State作者:Jörg Knizek、Heinrich Nöth、Markus WarchholdDOI:10.1515/znb-2006-0906日期:2006.9.1BH3 ·THF in THF solution. It is unstable to disproportionation. Compound (C6F5O)3B·THF was isolated and its crystal structure determined. Reaction of LiBH4 with F5C6OH in hexane generated a solid that proved to be Li[H2B(OC6F5)2]. It is unstable in THF. On the other hand, 2,2’-dihydroxydiphenyl in the presence of secondary amines reacts to give Li[C12H8O2B(NR2)2] (3 - 5). Li[B(O2C12H8)2], 2, is formed摘要 MBH4 (M = Li, Na, K) 与 tBuOH、Ph3COH、PhOH、F5C6OH 和 2,4-tBu2C6H3OH 在 THF 中以 1:1 的比例进行反应,然后进行 11B NMR 光谱分析。未检测到 M[H2B(OR)2] 物质,但检测到少量 M[H3BOR] 和大量 M[HB(OR)3]。在 LiBH4 与 2,4-tBu2C6H3OH 的反应中,也生成了相当比例的 (RO)2BH。全氟苯酚硼烷(F5C6O)2BH·THF由苯酚和BH3·THF在THF溶液中制备。对歧化不稳定。分离出化合物(C6F5O)3B·THF并确定其晶体结构。LiBH4 与 F5C6OH 在己烷中的反应生成固体,证明是 Li[H2B(OC6F5)2]。在 THF 中不稳定。另一方面,2,2'-二羟基二苯基在仲胺存在下反应生成 Li[C12H8O2B(NR2)2] (3 - 5)。Li[B(O2C12H8)2]
-
Controlling Singlet–Triplet Energy Splitting for Deep‐Blue Thermally Activated Delayed Fluorescence Emitters作者:Lin‐Song Cui、Hiroko Nomura、Yan Geng、Jong Uk Kim、Hajime Nakanotani、Chihaya AdachiDOI:10.1002/anie.201609459日期:2017.2metal‐free organic emitters with thermally activated delayed fluorescence (TADF) properties for deep‐blue emission is still challenging. A new family of deep‐blue TADF emitters based on a donor–acceptor architecture has been developed. The electronic interaction between donor and acceptor plays a key role in the TADF mechanism. Deep‐blue OLEDs fabricated with these TADF emitters achieved high external具有热激活延迟荧光(TADF)特性的深蓝色发射的高效无金属有机发射体的开发仍然具有挑战性。已经开发了基于施主-受主结构的深蓝色TADF发射器新系列。供体和受体之间的电子相互作用在TADF机制中起着关键作用。使用这些TADF发射器制造的深蓝色OLED的外部量子效率高达19.2%,CIE坐标为(0.148,0.098)。
-
METHOD FOR PREPARING BORINIC ACID DERIVATIVES AND NOVEL BORINIC ACID DERIVATIVES申请人:MANAC INC.公开号:US20150105562A1公开(公告)日:2015-04-16The present invention relates to a method for preparing borinic acid derivatives and novel borinic acid derivatives. The preparing method of the present invention provides borinic acid derivatives of general formula (2): (Ar 2 B(OH) (2) wherein Ar is the same as defined in the description and claims, selectively and in a high yield by reacting a compound of general formula (1): Ar-M, (1) wherein Ar and M are the same as defined in the description and claims, with tri-t-butyl borate and then hydrolyzing the reaction product.
-
一种5-(8-溴-2,3-二苯基喹喔啉-5-yl)噻吩- 2-甲醛的合成方法申请人:西安瑞联新材料股份有限公司公开号:CN106892908B公开(公告)日:2021-04-06
表征谱图
-
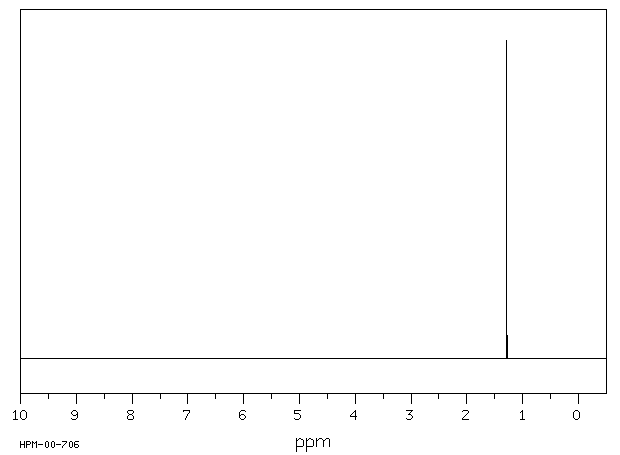
氢谱1HNMR
-
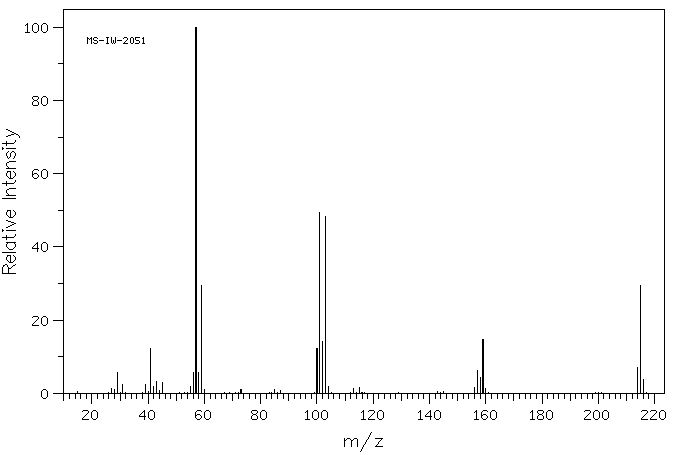
质谱MS
-
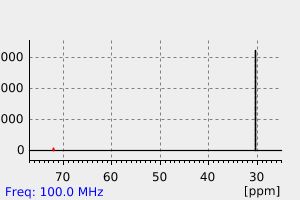
碳谱13CNMR
-
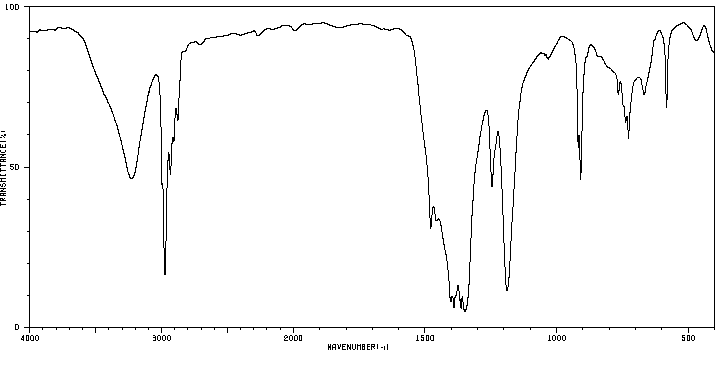
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高氯酸钪
高氯酸胍
高氯酸甲酯
高氯酸四甲基铵
高氯酸四乙基铵
高氯酸吡咯烷
高氯酸三丁基锡
高氯酸[4-(2,5-二羰基吡咯烷-1-基)丁-2-炔-1-基](二甲基)锍
高氯酸2-羟乙基酯
高氯酸2-羟丙基酯
顺式-2-硝基环己基高氯酸盐
铵钪硝酸盐(2:1:5)
铵氧代-三氧代锝
铅(2+)砷酸盐-三丁基锡烷基水合物(3:2:2:1)
钪三溴酸盐
钠O-(四氢-2-呋喃基甲基)硫代硫酸酯
重铬酸咪唑
重铬酸吡啶
过氧硝酸甲酯
过氧硝酸环戊酯
过氧化,硝基1-羰基-2-丙烯基
过氧化,甲磺酰硝基
过氧丙酰硝酸酯
膦酸二异丁基酯
磷酸组胺
磷酸(羟基-甲氧基-磷酰)二甲酯
磷酸(羟基-异戊氧基磷酰)二异戊基酯
碳化钨
碲烷
碲杂环丁烷
碲吩-2-羧酸
碲吩
硼酸异辛酯
硼酸十六烷基酯
硼酸三辛酯
硼酸三甲酯
硼酸三烯丙酯
硼酸三正己酯
硼酸三月桂酯
硼酸三异丙酯
硼酸三庚酯
硼酸三亚甲酯
硼酸三乙酯
硼酸三丙酯
硼酸三丁酯-11B
硼酸三丁酯-10B
硼酸三丁酯
硼酸三-[3-(3-甲氧基-丙氧基)-丙基]酯
硼酸三-(2-氯甲氧基-乙基)酯
硼酸三(六氟异丙基)酯