辛-2,7-二烯-1-醇 | 79972-62-6
中文名称
辛-2,7-二烯-1-醇
中文别名
——
英文名称
(2Z)-octa-2,7-dien-1-ol
英文别名
(Z)-octa-2,7-dien-1-ol;(Z)-2,7-octadien-1-ol
CAS
79972-62-6
化学式
C8H14O
mdl
——
分子量
126.199
InChiKey
YHYGSIBXYYKYFB-SREVYHEPSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:96 °C(Press: 12 Torr)
-
密度:0.859±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:9
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
反应信息
-
作为反应物:描述:辛-2,7-二烯-1-醇 生成 [(1R,5S,6R)-6-bicyclo[3.2.0]heptanyl]methanol参考文献:名称:SALOMON, R. G.;COUGHLIN, D. J.;GHOSH, S.;ZAGORSKI, M. G., J. AMER. CHEM. SOC., 1982, 104, N 4, 998-1007摘要:DOI:
-
作为产物:描述:参考文献:名称:非质子介质中2,3-环氧醇的受控佩恩重排:(+)- exo -brevicomin的对映选择性全合成摘要:描述了氯化锂在四氢呋喃溶液中催化顺式-2,3-环氧伯醇异构化为苏-1,2-环氧醇的反应。通过与亲核试剂反应可以将更多反应性的末端环氧化物选择性地原位捕获,并且该方法用于(+)- exo- brevicomin的对映选择性合成。DOI:10.1039/c39880000356
文献信息
-
Preparation of Oxocanes by Electrophilic Cyclizations of Unsaturated Alcohols in the Presence of Bis(collidine)halonium(I) Hexafluorophosphates作者:Christelle Mendès、Sylvie Renard、Mazin Rofoo、Marie-Claude Roux、Gérard RousseauDOI:10.1002/ejoc.200390080日期:2003.2carbon chain with carbocyclic (phenyl and cyclopropane) and heterocyclic (epoxide and dioxolane) moieties were prepared and their cyclizations in the presence of bis(collidine)iodonium(I) and -bromonium(I) hexafluorophosphates as electrophiles were studied. Oxocanes were obtained in modest to good yields when a rigid cyclic moiety (cyclopropane or phenyl) was present in the chain, while yields of cyclizations
-
Total Synthesis of Leukotrienes from Butadiene作者:Ana Rodríguez、Miguel Nomen、Bernd W. Spur、Jean-Jacques Godfroid、Tak H. LeeDOI:10.1002/1099-0690(200009)2000:17<2991::aid-ejoc2991>3.0.co;2-h日期:2000.9The total synthesis of leukotrienes has been achieved starting from butadiene by a palladium-catalyzed telomerization at room temperature. A Sharpless catalytic asymmetric epoxidation generated the asymmetric centers with >94% ee. Simple transformations of the key intermediate 15 produced the leukotrienes LTA4 methyl ester (4), LTC4 (1), LTD4 (2) and LTE4 (3), as well as (14S,15S)-LTA4 methyl ester (24)
-
Zirconium-Mediated S<sub>N</sub>2‘ Substitution of Allylic Ethers: Regio- and Stereospecific Formation of Protected Allylic Amines作者:Gojko Lalic、Suzanne A. Blum、Robert G. BergmanDOI:10.1021/ja056132f日期:2005.12.1A new zirconium-mediated, regio- and stereospecific SN2' substitution of allylic ethers with a nitrogen nucleophile has been developed. Cbz-protected amine products were isolated in high yield from reactions with a wide range of Z allylic ethers. A mechanism of the allylic substitution consistent with the results of the kinetics and kinetic isotope effect studies was proposed.
-
Scope and Mechanism of Formal S<sub>N</sub>2‘ Substitution Reactions of a Monomeric Imidozirconium Complex with Allylic Electrophiles作者:Gojko Lalic、Jamin L. Krinsky、Robert G. BergmanDOI:10.1021/ja7106096日期:2008.4.1bromides to give exclusively the products of the SN2' reaction; i.e., attack at the allylic position remote from the leaving group with migration of the double bond. The primary amine products can be isolated in excellent yields, after in situ Cbz protection, in the presence of variety of functional groups. Good diastereoselectivity and complete stereoselectivity allowed the formation of enantioenriched锆亚胺配合物 Cp2(THF)Zr=NSi(t-Bu)Me2 (1) 与烯丙基醚、氯化物和溴化物反应,仅生成 SN2' 反应的产物;即,随着双键的迁移,在远离离去基团的烯丙基位置处进行攻击。在各种官能团的存在下,在原位 Cbz 保护后,可以以极好的收率分离伯胺产物。良好的非对映选择性和完全立体选择性允许从富含对映体的烯丙基醚形成富含对映体的烯丙基胺。烯丙基氟化物也实现了 1 的区域特异性取代,众所周知,烯丙基氟化物是其他取代反应中的不良底物。在速率和动力学同位素效应研究的基础上,我们提出了与 1 的烯丙基取代反应的一般机制,其中涉及 THF 的解离和底物的结合,然后是取代步骤。在取代反应的 DFT 研究中,我们确定了取代步骤的六元闭合过渡态和沿反应坐标的其他相关驻点。该研究表明,取代反应可以描述为协调的异步 [3,3]-σ 重排。对反应机理的详细了解为观察到的区域选择性、非对映选择性和立体
-
Copper(I) catalysis of olefin photoreactions. 9. Photobicyclization of .alpha.-, .beta.-, and .gamma.-alkenylallyl alcohols作者:Robert G. Salomon、Daniel J. Coughlin、Subrata Ghosh、Michael G. ZagorskiDOI:10.1021/ja00368a014日期:1982.2
表征谱图
-
氢谱1HNMR
-
质谱MS
-
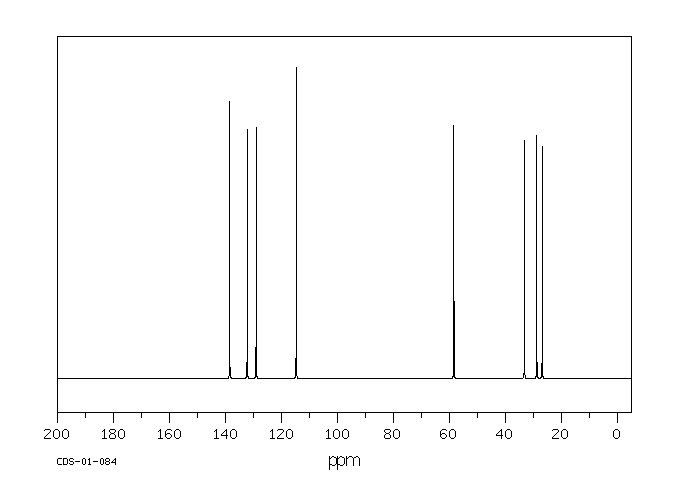
碳谱13CNMR
-
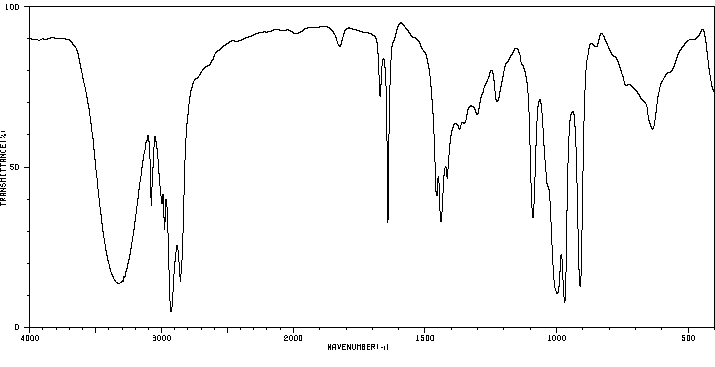
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯