二氢-2H-硫代吡喃-3(4h)-酮 | 19090-03-0
中文名称
二氢-2H-硫代吡喃-3(4h)-酮
中文别名
二氢-2H-四氢吡喃-3-酮
英文名称
dihydro-2H-thiopyran-3(4H)-one
英文别名
tetrahydrothiopyran-3-one;tetrahydro-4H-thiopyran-3-one;thian-3-one
CAS
19090-03-0
化学式
C5H8OS
mdl
MFCD00040796
分子量
116.184
InChiKey
ATAMXDLUUTYFKT-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:80 °C(Press: 4 Torr)
-
密度:1.134±0.06 g/cm3(Predicted)
-
保留指数:1541
计算性质
-
辛醇/水分配系数(LogP):0.8
-
重原子数:7
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.8
-
拓扑面积:42.4
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2934999090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:2-8°C
SDS
上下游信息
反应信息
-
作为反应物:描述:二氢-2H-硫代吡喃-3(4h)-酮 在 sodium tetrahydroborate 、 二溴二氟甲烷 、 palladium 10% on activated carbon 、 水 、 氢气 、 二异丁基氢化铝 、 三乙基硼氢化锂 、 1,8-二氮杂双环[5.4.0]十一碳-7-烯 、 三乙胺 、 间氯过氧苯甲酸 、 叔丁醇 作用下, 以 四氢呋喃 、 甲醇 、 正庚烷 、 二氯甲烷 为溶剂, -78.0~60.0 ℃ 、200.0 kPa 条件下, 反应 12.58h, 生成 3-甲基-1-环己烯参考文献:名称:通过 Ir 催化不对称加氢对映选择性合成手性砜:制备手性烯丙基和均烯丙基化合物的简便方法摘要:开发了一种高效且对映选择性 Ir 催化的不饱和砜加氢反应。以优异的对映选择性(高达 98% ee)生产手性环状和无环砜。与 Ramberg-Bäcklund 重排相结合,该反应提供了一种获得具有优异对映选择性(高达 97% ee)和高产率(高达 94%)的手性烯丙基和同型烯丙基化合物的新途径。DOI:10.1021/ja306731u
-
作为产物:描述:alkaline earth salt of/the/ methylsulfuric acid 在 硫酸 作用下, 生成 二氢-2H-硫代吡喃-3(4h)-酮参考文献:名称:Thiapyran Derivatives. III. The Preparation, Properties and Reactions of ▵2-Dihydrothiapyran 1,1-Dioxide摘要:DOI:10.1021/ja01126a068
文献信息
-
[EN] NOVEL COMPOUNDS<br/>[FR] NOUVEAUX COMPOSÉS申请人:GLAXOSMITHKLINE IP DEV LTD公开号:WO2015180612A1公开(公告)日:2015-12-03Disclosed are novel retinoid-related orphan receptor gamma (RORγ) modulators and their use in the treatment of diseases mediated by RORγ.揭示了新型视黄醇相关孤儿受体γ(RORγ)调节剂及其在通过RORγ介导的疾病治疗中的应用。
-
Synthese und pharmakologische Prüfung ZNS-wirksamer Phenyl-tetrahydropyrane und -thiopyrane: Oxa- und Thia-phencyclidine作者:Fritz Eiden、Michael Schmidt、Helga BuchbornDOI:10.1002/ardp.19873200413日期:——Aus den Pyranonen 2 und 14 sowie den Thiopyranonen 3 und 15 wurden Phencyclidin‐oxa‐ und ‐thiaanaloge dargestellt. An Mäusen zeigten die Pyranderivate ein günstigeres Verhältnis von Analgesie und Erregung als Phencyclidin. Während die analgetischen Eigenschaften der Enantiomere (+)‐20b und (−)‐20b gleich waren, ließ sich eine erregende Wirkung bei (+)‐20b nicht mehr feststellen. Die Thiopyranderivate
-
[1,2,4]Triazino[2,3-с]quinazolines 2*. Synthesis, structure, and anticonvulsant activity of new 3′-R1-spiro[(aza/oxa/thia)cycloalkyl-1(3, 4),6′-[1,2,4]triazino[2,3-c]quinazolin]-2′(7′H)-ones作者:Oleksii Yu. Voskoboynik、Olexandra S. Kolomoets、Vitaliy A. Palchikov、Sergyi I. Kovalenko、Igor F. Belenichev、Svetlana V. ShishkinaDOI:10.1007/s10593-017-2184-8日期:2017.10Previously unknown 3′-R1-spiro[(aza/oxa/thia)cycloalkyl-1(3, 4),6′-[1,2,4]triazino[2,3-c]quinazolin]-2′(7′H)-ones were obtained on the basis of (5+1) cyclocondensation reaction of substituted 6-R1-3-(2-aminophenyl)-1,2,4-triazin-5(2Н)-ones with cyclic ketones. It was established that the steric and electronic factors of cyclic ketones did not affect the reaction duration and product yields. The structure
-
Aldol additions of pinacolone lithium enolate with ketones: reactivities governed predominantly by field effects作者:Goutam Das、Edward R. ThorntonDOI:10.1021/ja00057a012日期:1993.2The relative reactivities of representative α- and β-heterosubstituted acyclic, cyclic (five- and six-membered), and aromatic ketones with the lithium enolate of pinacolone in diethyl ether at -78 o C were determined. The order of reactivitiesof monosubstituted acetones (MeCOCH 2 X) is X=Cl>OTBDMS>OMe>SMe>NMe 2 >CH 2 SM>H>Me and spans a range of 10 4
-
Multigram scale synthesis of 3,4- and 3,6-dihydro-2<i>H</i>-thiopyran 1,1-dioxides and features of their NMR spectral behavior作者:Roman M. Chabanenko、Svitlana Yu. Mykolenko、Eugene K. Kozirev、Vitalii A. PalchykovDOI:10.1080/00397911.2018.1486427日期:2018.9.2Abstract A new four-step synthesis of 3,4- and 3,6-dihydro-2H-thiopran-1,1-dioxides from dihydro-2H-thiopyran-3(4H)-one is reported. The title compounds are synthesized starting with oxidation of the ketone with a 30% aqueous solution of hydrogen peroxide in a mixture of AcOH-Ac2O. The keto group is then reduced by sodium borohydride followed by mesylation and elimination of methanesulfonic acid under摘要 报道了一种从二氢-2H-噻喃-3(4H)-one 合成 3,4- 和 3,6-dihydro-2H-thiopran-1,1-dioxides 的新四步法。标题化合物的合成开始于酮用 30% 过氧化氢水溶液在 AcOH-Ac 2 O 的混合物中的氧化。然后用硼氢化钠还原酮基,然后在碱性条件下甲磺酸化和消除甲磺酸(3,4-异构体为吡啶,3,6-异构体为NaOH水溶液)。该序列比以前已知的方法更简单,使用更便宜且更容易获得的试剂,并产生多克规模的 2H-thiopran-1,1-二氧化物,总产率分别为 64% 和 74%。化合物的结构和纯度通过2D NMR和GCMS方法确认。所提出的方法扩展了访问功能化环砜作为合成新生物活性化合物组合库的构建模块的方法。图形概要
表征谱图
-
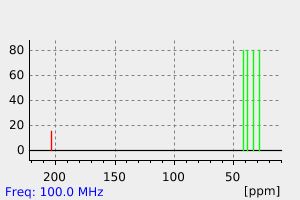
氢谱1HNMR
-
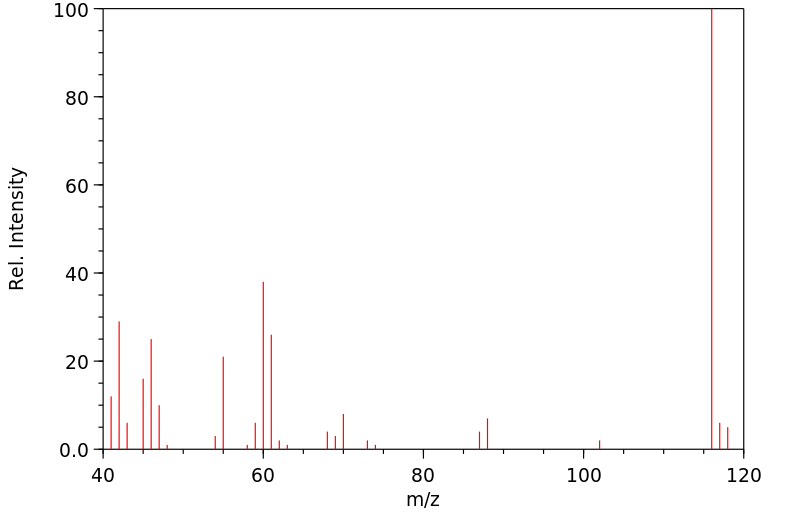
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
阿普卡林
硫化环戊烷
硫代环己酮
甲基硫酸四氢1-乙基-2-[1,2,3,4--1-(2-羟基乙基)-2,2,4-三甲基-6-喹啉基]苯[cd]吲哚正离子
甲基(1,1-二氧化四氢-2h-噻喃-4-基)醋酸盐
外-3-乙酰基-2-硫杂二环<2.2.2>辛-5-烯
四氢硫代吡喃-4-胺盐酸盐
四氢硫代吡喃-4-羰酰氯
四氢硫代吡喃-4-羧酸甲酯
四氢硫代吡喃-4-甲腈
四氢硫代吡喃-4-基甲醇
四氢硫代吡喃-3-甲醛
四氢噻喃-4-醇
四氢噻喃-4-酮肟
四氢噻喃-4-酮 1,1-二氧化物
四氢噻喃-4-酮
四氢噻喃-4-胺
四氢噻喃-4-肼双盐酸盐
四氢噻喃-4-甲醛
四氢-4H-硫代吡喃-4-酮 1-氧化物
四氢-4-氧代-2H-噻喃-3-甲酸甲酯
四氢-3-甲基-2H-噻喃
四氢-3-氧代-6H-噻喃-2-甲酸甲酯
四氢-2H-硫代吡喃-4-醇 1,1-二氧化物
四氢-2H-硫代吡喃-4-羧醛 1,1-二氧化物
四氢-2H-硫代吡喃-4-甲醇 1,1-二氧化物
四氢-2H-硫代吡喃-4-乙醛
四氢-2H-噻喃-4-羧酸甲酯1,1-二氧化物
四氢-2H-噻喃-4-甲酰肼
四氢-2H-噻喃-4-甲腈1,1-二氧化
四氢-2H-噻喃-3-醇1,1-二氧化物
四氢-2H-噻喃-3-羧酸1,1-二氧化物
四氢-(9ci)-2H-硫代吡喃-4-羧酸
噻-4-基甲胺
叔-丁基[(1S,2R)-1-苯甲基-2-羟基-3-[异丁基[(4-硝基苯基)磺酰]氨基]丙基]氨基甲酸酯
二氢-5,5-二甲基-2H-硫基吡喃-3(4H)-酮-1,1-二氧化物
二氢-2H-硫代吡喃-3(4h)-酮
二氢-2H-硫代吡喃-3(4H)-酮-1,1-二氧化物
乙酸四氢-2H-噻喃-2-基酯
三环己基乙基硼酸钠
n-[四氢-2H-硫代吡喃-4-基]氨基甲酸-1,1-二甲基乙酯
N-甲基四氢-2H-硫代吡喃-4-胺盐酸盐
N-甲基四氢-2H-噻喃-4-胺盐酸盐
N-甲基(四氢硫代吡喃-4-基)甲基胺
9-硫杂二环[3.3.1]壬烷-2,6-二酮
9-硫杂二环[3.3.1]壬烷
8-硫杂二环[3.2.1]辛烷-3-酮
8-硫杂-2,3-二氮杂螺[4.5]癸烷
8-乙烯基-7-硫杂-二环[4.2.0]辛烷
7-硫杂-2-氮杂螺[3.5]壬烷半草酸酯