4-(tert-butyl)-4'-iodo-1,1'-biphenyl
中文名称
——
中文别名
——
英文名称
4-(tert-butyl)-4'-iodo-1,1'-biphenyl
英文别名
4-tert-butyl-4'-iodobiphenyl;1-tert-butyl-4-(4-iodophenyl)benzene
CAS
——
化学式
C16H17I
mdl
——
分子量
336.215
InChiKey
HPIHETXXQCHXAU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):5.9
-
重原子数:17
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
上下游信息
反应信息
-
作为反应物:描述:4-(tert-butyl)-4'-iodo-1,1'-biphenyl 在 palladium on activated charcoal 18-冠醚-6 、 氢气 、 铜 、 potassium carbonate 作用下, 以 四氢呋喃 、 邻二氯苯 为溶剂, 反应 72.0h, 生成 4-(N,N-bis(4-tert-butylbiphenyl-4'-yl)amino)aniline参考文献:名称:Syntheses of [60]Fullerene andN,N-Bis(4-biphenyl)aniline-Tethered Rotaxane: Photoinduced Electron-Transfer Processes via Singlet and Triplet States of [60]Fullerene摘要:我们合成了一种含有[60]富勒烯(C60)和 N,N-双(4-联苯)苯胺(BBA)分子的轮烷。在这种结构中,作为电子受体的 C60 连接到冠醚环上,而两端作为电子供体的末端 BBA 分子穿过冠醚环的轴。这种轮烷的轴中心有一个中性酰胺分子,其中两个 BBA 分子起阻挡作用。在改变溶剂极性和温度的情况下,通过时间分辨瞬态吸收和荧光测量,研究了 C60 和 BBA 分子在轮烷内部光诱导的电子转移过程。轮烷的时间分辨瞬态吸收测量结果证实,在极性溶剂中,长寿命电荷分离态(C60--; BBA-+)轮烷是通过 C60 的激发单线态和三线态(分别为 1C60* 和 3C60*)形成的。经评估,通过 1C60* 和 3C60* 电荷分离过程的速率常数分别为 (3.6-3.7) × 108 s-1 和 (5.1-5.6) × 107 s-1,其比例为 (0.36-0.38): (0.43-0.51)。电荷重组的速率常数分别为 2.5 × 106 s-1 和 4.4 × 106 s-1,对应于电荷分离态在 THF 和苯甲腈中的寿命分别为 400 ns 和 230 ns。根据温度相关性,通过 3C60* 进行电荷分离过程的活化自由能变化被估算为 0.10 eV,而在 THF 和苯甲腈中进行电荷重组过程的活化自由能变化被估算为 0.03 eV。这些低活化能是轮烷中通过空间电子转移的特征之一。DOI:10.1246/bcsj.78.1008
-
作为产物:参考文献:名称:Creation of azobenzene-based photochromic amorphous molecular materials—synthesis, glass-forming properties, and photochromic response摘要:创建了一系列新型偶氮苯基光致变色无定形分子材料。人们发现它们很容易形成具有明确玻璃化转变温度的非晶玻璃,并且在非晶薄膜和溶液中都表现出光致变色性。研究发现,非晶态薄膜的反式-顺式光异构化量子产率小于溶液中的量子产率,并且非晶态薄膜的逆式顺式-反式热异构化反应相对于溶液中的反应加速或延迟,具体取决于它们的分子结构。此外,随着生成顺式异构体的照射时间变短,非晶态薄膜相对于溶液的顺式-反式热异构化的速率加速更加显着。DOI:10.1039/b711542c
文献信息
-
Visible‐Light‐Induced [4+2] Annulation of Thiophenes and Alkynes to Construct Benzene Rings作者:Chunlan Song、Xin Dong、Zhongjie Wang、Kun Liu、Chien‐Wei Chiang、Aiwen LeiDOI:10.1002/anie.201905971日期:2019.8.26The [4+2] annulation represents an elegant and versatile synthetic protocol for the construction of benzene rings. Herein, a strategy for visible-light induced [4+2] annulation of thiophenes and alkynes, to afford benzene rings, is presented. Under simple and mild reaction conditions, the ready availability and structural diversity of thiophenes and alkynes permit the facile synthesis of several substituted
-
Spatial Anion Control on Palladium for Mild C–H Arylation of Arenes作者:Jyoti Dhankhar、Elisa González-Fernández、Chao-Chen Dong、Tufan K. Mukhopadhyay、Anthony Linden、Ilija ČorićDOI:10.1021/jacs.0c09611日期:2020.11.11C-H arylation of arenes without the use of directing groups is a challenge, even for simple molecules, such as benzene. We describe spatial anion control as a concept for the design of catalytic sites for C-H bond activation, thereby enabling nondirected C-H arylation of arenes at ambient temperature. The mild conditions enable late-stage structural diversification of biologically relevant small molecules
-
Palladium‐Catalyzed Secondary C( <i>sp</i> <sup>3</sup> )−H Arylation of 2‐Alkylpyridines作者:Hong‐Liang Li、Yoichiro KuninobuDOI:10.1002/adsc.202000306日期:2020.7.16A pyridyl group‐assisted palladium‐catalyzed secondary C(sp 3)−H arylation protocol was developed. A substituent at the 3‐position of the pyridyl group is proved to be important for promoting C−H arylation and controlling the regioselectivity. Aryl iodides can be used as coupling partners. The reaction proceeded in good to excellent yields with good functional group tolerance, even on the gram‐scale
-
Palladium-Catalyzed Enantioselective C(sp<sup>3</sup>)–H Arylation of 2-Propyl Azaaryls Enabled by an Amino Acid Ligand作者:Hong-Liang Li、Deng-Feng Yang、Hua-Qing Jing、Jon C. Antilla、Yoichiro KuninobuDOI:10.1021/acs.orglett.1c04215日期:2022.2.18A palladium(II)-catalyzed enantioselective arylation of unbiased secondary C(sp3)–H bonds was developed. The enantioselectivity was controlled by the combination of a pyridyl or isoquinolinyl directing group and an amino acid, N-Boc-2-pentyl proline. A variety of 2-propyl azaaryls and biaryl iodides were employed to provide arylated products in moderate to good yields (up to 82%) with high enantioselectivities
-
4-[Di(biphenyl-4-yl)amino]azobenzene and 4,4′-bis[bis(4′-tert-butylbiphenyl-4-yl)amino]azobenzene as a novel family of photochromic amorphous molecular materials作者:Yasuhiko Shirota、Kazuyuki Moriwaki、Satoru Yoshikawa、Toshiki Ujike、Hideyuki NakanoDOI:10.1039/a806802j日期:——We have created photochromic amorphous molecular materials containing an azobenzene chromophore, 4-[di(biphenyl-4-yl)amino]azobenzene and 4,4â²-bis[bis(4â²-tert-butylbiphenyl-4-yl)amino]azobenzene (t-BuBBAB), which readily form amorphous glasses above room temperature and exhibit photochromism in their amorphous films. We have shown that the photogenerated cis-form in the amorphous film can be stabilized at ambient temperature by the incorporation of a bulky group; the backward cisâtrans thermal isomerization can be controlled by temperature for t-BuBBAB.
表征谱图
-
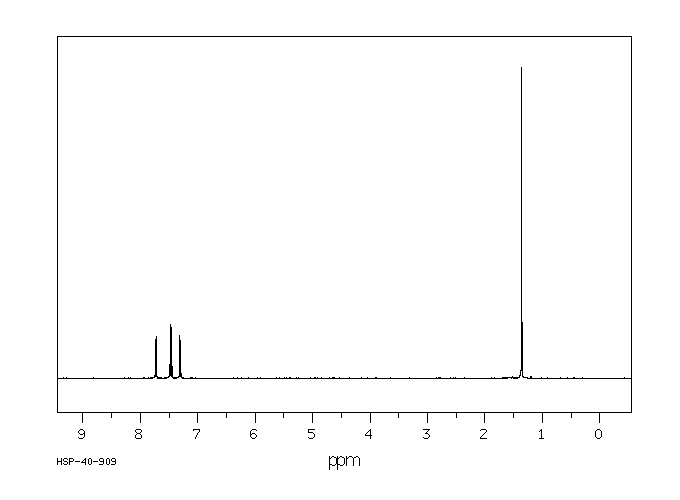
氢谱1HNMR
-
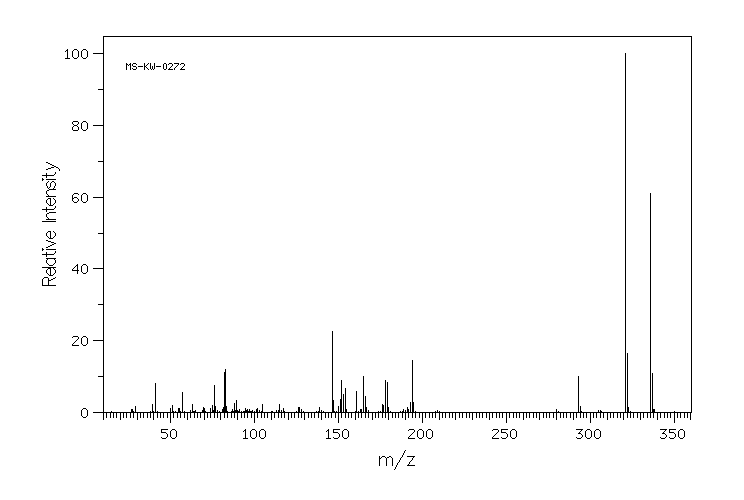
质谱MS
-
碳谱13CNMR
-
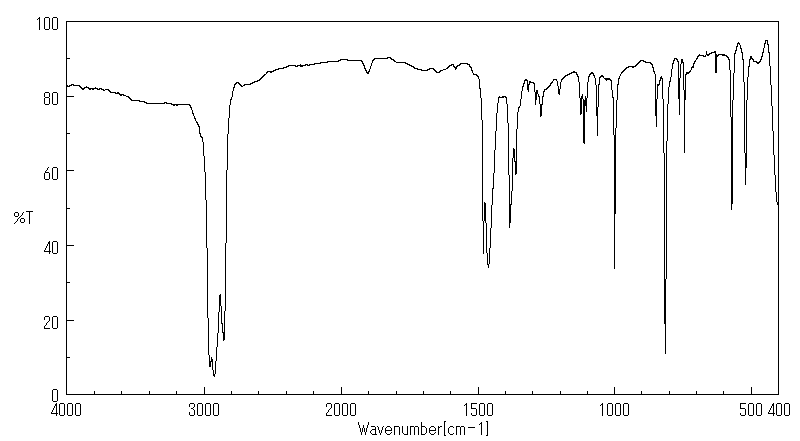
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫