(3,3-二甲基丁基)苯 | 17314-92-0
中文名称
(3,3-二甲基丁基)苯
中文别名
——
英文名称
(3,3-dimethylbutyl)benzene
英文别名
1-phenyl-3,3-dimethylbutane;tert-butyl ethylbenzene;3,3-dimethylbutylbenzene
CAS
17314-92-0
化学式
C12H18
mdl
MFCD03701472
分子量
162.275
InChiKey
NKDJZTYRVIGJCG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):4.9
-
重原子数:12
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— (3-fluoro-3-methylbutyl)benzene 19031-66-4 C11H15F 166.239 3,3-二甲基-1-苯基-2-丁酮 tert-butyl benzyl ketone 6721-67-1 C12H16O 176.258 苄基丙酮 4-Phenyl-2-butanone 2550-26-7 C10H12O 148.205 3,3-二甲基-1-苯基-1-丁酮 3,3-dimethyl-1-phenyl-butan-1-one 31366-07-1 C12H16O 176.258
反应信息
-
作为反应物:描述:(3,3-二甲基丁基)苯 在 sodium hydroxide 、 三氯化铝 、 一水合肼 作用下, 以 二硫化碳 为溶剂, 反应 34.0h, 生成 1,4-dineohexyl-2,3,5,6-tetraethylbenzene参考文献:名称:Effect of transition-metal complexation on the stereodynamics of persubstituted arenes. Evidence for steric complementarity between arene and metal tripod摘要:The stereodynamics in 1,4-dimethoxy-2,3,5,6-tetraethylbenzene (5), 1,4-bis(methoxymethyl)-2,3,5,6-tetraethylbenzene (6), and 1,4-dineohexyl-2,3,5,6-tetraethylbenzene (7) and their respective tricarbonylchromium complexes, 5(Cr), 6(Cr), and 7(Cr), have been studied by variable-temperature NMR techniques. Barriers to rotation about the sp2-sp3 bonds for 5-7 and 5(Cr)-7(Cr) have been determined using the Gutowsky-Holm approximation to be 7.7, 9.4, 11.2, 6.6, 8.9, and 11.8 kcal/mol, respectively. Unlike previous studies in this area, the stereodynamics of the arene do not change demonstrably upon metal complexation. This observation is attributed to a lock-and-key complementarity between the metal tripod and the arene. The possibility of correlated dynamics between the metal tripod rotation and the ethyl group rotation is discussed.DOI:10.1021/ja00027a033
-
作为产物:描述:3,3-二甲基-1-苯基-1-丁酮 在 sodium hydroxide 、 一水合肼 作用下, 以 various solvent(s) 为溶剂, 反应 4.0h, 以75%的产率得到(3,3-二甲基丁基)苯参考文献:名称:Effect of transition-metal complexation on the stereodynamics of persubstituted arenes. Evidence for steric complementarity between arene and metal tripod摘要:The stereodynamics in 1,4-dimethoxy-2,3,5,6-tetraethylbenzene (5), 1,4-bis(methoxymethyl)-2,3,5,6-tetraethylbenzene (6), and 1,4-dineohexyl-2,3,5,6-tetraethylbenzene (7) and their respective tricarbonylchromium complexes, 5(Cr), 6(Cr), and 7(Cr), have been studied by variable-temperature NMR techniques. Barriers to rotation about the sp2-sp3 bonds for 5-7 and 5(Cr)-7(Cr) have been determined using the Gutowsky-Holm approximation to be 7.7, 9.4, 11.2, 6.6, 8.9, and 11.8 kcal/mol, respectively. Unlike previous studies in this area, the stereodynamics of the arene do not change demonstrably upon metal complexation. This observation is attributed to a lock-and-key complementarity between the metal tripod and the arene. The possibility of correlated dynamics between the metal tripod rotation and the ethyl group rotation is discussed.DOI:10.1021/ja00027a033
文献信息
-
Facile Regio- and Stereoselective Hydrometalation of Alkynes with a Combination of Carboxylic Acids and Group 10 Transition Metal Complexes: Selective Hydrogenation of Alkynes with Formic Acid作者:Ruwei Shen、Tieqiao Chen、Yalei Zhao、Renhua Qiu、Yongbo Zhou、Shuangfeng Yin、Xiangbo Wang、Midori Goto、Li-Biao HanDOI:10.1021/ja2069246日期:2011.10.26highly stereo- and regioselective hydrometalation of alkynes generating alkenylmetal complex is disclosed for the first time from a reaction of alkyne, carboxylic acid, and a zerovalent group 10 transition metal complex M(PEt(3))(4) (M = Ni, Pd, Pt). A mechanistic study showed that the hydrometalation does not proceed via the reaction of alkyne with a hydridometal generated by the protonation of a
-
Tetrahydroxydiboron-Mediated Palladium-Catalyzed Transfer Hydrogenation and Deuteriation of Alkenes and Alkynes Using Water as the Stoichiometric H or D Atom Donor作者:Steven P. Cummings、Thanh-Ngoc Le、Gilberto E. Fernandez、Lorenzo G. Quiambao、Benjamin J. StokesDOI:10.1021/jacs.6b02132日期:2016.5.18There are few examples of catalytic transfer hydrogenations of simple alkenes and alkynes that use water as a stoichiometric H or D atom donor. We have found that diboron reagents efficiently mediate the transfer of H or D atoms from water directly onto unsaturated C-C bonds using a palladium catalyst. This reaction is conducted on a broad variety of alkenes and alkynes at ambient temperature, and boric
-
Iron-catalysed, hydride-mediated reductive cross-coupling of vinyl halides and Grignard reagents作者:Bryden A. F. Le Bailly、Mark D. Greenhalgh、Stephen P. ThomasDOI:10.1039/c1cc14622j日期:——hydride-mediated reductive cross-coupling reaction has been developed for the preparation of alkanes. Using a bench-stable iron(II) pre-catalyst, reductive cross-coupling of vinyl iodides, bromides and chlorides with aryl- and alkyl Grignard reagents successfully gave the products of formal sp(3)-sp(3) cross-coupling reactions.
-
Catalytic Synthesis of “Super” Linear Alkenyl Arenes Using an Easily Prepared Rh(I) Catalyst作者:Michael S. Webster-Gardiner、Junqi Chen、Benjamin A. Vaughan、Bradley A. McKeown、William Schinski、T. Brent GunnoeDOI:10.1021/jacs.7b01165日期:2017.4.19production of 1-phenyl substituted alkene products via oxidative arene vinylation. Since C═C bonds can be used for many chemical transformations, the formation of unsaturated products provides a potential advantage over current processes that produce saturated alkyl arenes. Conditions that provide up to a 10:1 linear:branched ratio have been achieved, and catalytic turnovers >1470 have been demonstrated
-
Iron-catalysed alkene hydrogenation and reductive cross-coupling using a bench-stable iron(ii) pre-catalyst作者:Dominik J. Frank、Léa Guiet、Alexander Käslin、Elliot Murphy、Stephen P. ThomasDOI:10.1039/c3ra44519d日期:——Operationally simple, iron-catalysed hydrogenation and reductive cross-coupling protocols have been developed using a bench-stable iron(II) pre-catalyst. The hydrogenation of 18 alkenes (50–99%) and reductive cross-coupling of vinyl halides with aryl- and alkyl Grignard reagents (8 examples, 18–99%) is reported using 3 mol% pre-catalyst and hydrogen as stoichiometric reductant (1–50 bar).
表征谱图
-
氢谱1HNMR
-
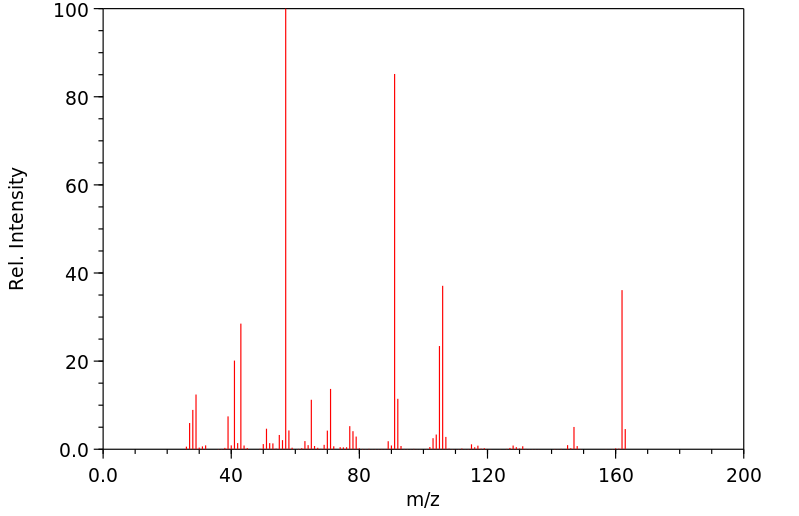
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫