(E)-庚-3-烯酸 | 28163-84-0
中文名称
(E)-庚-3-烯酸
中文别名
2-丙烯酸,2-氰基-,2,2,3,3,4,4,5,5-八氟戊基酯
英文名称
(E)-hept-3-enoic acid
英文别名
(E)-3-heptenoic acid;3-Heptenoic acid
CAS
28163-84-0
化学式
C7H12O2
mdl
——
分子量
128.171
InChiKey
BCYXFRMDZWKZSF-SNAWJCMRSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:77 °C(Press: 0.5 Torr)
-
密度:0.968±0.06 g/cm3(Predicted)
-
LogP:1.854 (est)
计算性质
-
辛醇/水分配系数(LogP):1.7
-
重原子数:9
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:0.57
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
WGK Germany:3
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (E)-ethyl 3-heptenoate 54340-71-5 C9H16O2 156.225 —— trans-Hept-3-en-1-al 21662-18-0 C7H12O 112.172 反式-3-庚烯-1-醇 trans-3-hepten-1-ol 2108-05-6 C7H14O 114.188
反应信息
-
作为反应物:描述:参考文献:名称:Schmidt, Justus Liebigs Annalen der Chemie, 1889, vol. 255, p. 79摘要:DOI:
-
作为产物:描述:参考文献:名称:Indane-1,2,3-trione ; a highly reactive enophile摘要:DOI:10.1016/s0040-4039(00)87116-6
文献信息
-
Enantiodivergent and γ-Selective Asymmetric Allylic Amination作者:Jianmin Wang、Jie Chen、Choon Wee Kee、Choon-Hong TanDOI:10.1002/anie.201107317日期:2012.3.5judicious choice of the double bond geometry of the the β,γ‐unsaturated carbonyl compound. Computational studies reveal the possible origin of the inversed enantioselectivity, and the potential for enantiodivergent synthesis chiral amine‐containing substrates is attractive.双重试剂:通过明智地选择β,γ-不饱和羰基化合物的双键几何结构,使用胍催化剂1进行的标题反应可提供具有优异对映选择性的两种对映异构体。计算研究表明,对映选择性反演的可能起源,对映异构合成手性含胺底物的潜力是诱人的。
-
Regioselective Ni-Catalyzed Carboxylation of Allylic and Propargylic Alcohols with Carbon Dioxide作者:Yue-Gang Chen、Bin Shuai、Cong Ma、Xiu-Jie Zhang、Ping Fang、Tian-Sheng MeiDOI:10.1021/acs.orglett.7b01208日期:2017.6.2efficient Ni-catalyzed reductive carboxylation of allylic alcohols with CO2 has been successfully developed, providing linear β,γ-unsaturated carboxylic acids as the sole regioisomer with generally high E/Z stereoselectivity. In addition, the carboxylic acids can be generated from propargylic alcohols via hydrogenation to give allylic alcohol intermediates, followed by carboxylation. A preliminary mechanistic
-
Efficient Pd-Catalyzed Regio- and Stereoselective Carboxylation of Allylic Alcohols with Formic Acid作者:Ming-Chen Fu、Rui Shang、Wan-Min Cheng、Yao FuDOI:10.1002/chem.201701971日期:2017.7.3Formic acid is efficiently used as a C1 source to directly carboxylate allylic alcohols in the presence of a low loading of palladium catalyst and acetic anhydride as additive to afford β,γ‐unsaturated carboxylic acids with excellent chemo‐, regio‐, and stereoselectivity. The reaction proceeds through a carbonylation process with in situ‐generated carbon monoxide under mild conditions, avoiding the
-
Taming the Carboxyl Group for Directed Carbometalation-Observations on the Use of Anions, Dianions and Ester Enolates作者:Sandy Desrat、Philip J. Gray、Matthew R. Penny、William B. MotherwellDOI:10.1002/chem.201403294日期:2014.6.23Carboxylate anions, dianions and ester enolates provide simultaneous protection and activation for directed carbometalation reactions. Advantage can be taken of the bis‐carbanionic character of the intermediate for further controlled CC bond forming reactions.
-
一种α,β-不饱和-γ-内酯的制备方法
表征谱图
-
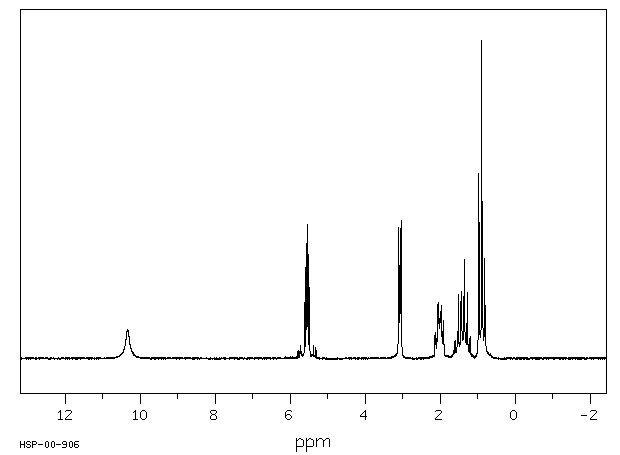
氢谱1HNMR
-
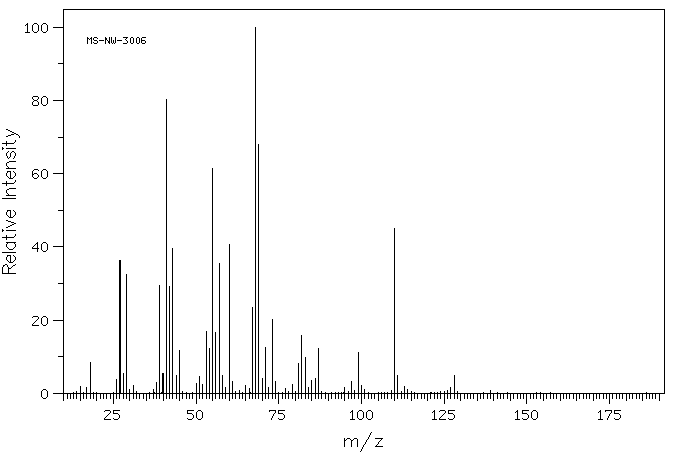
质谱MS
-
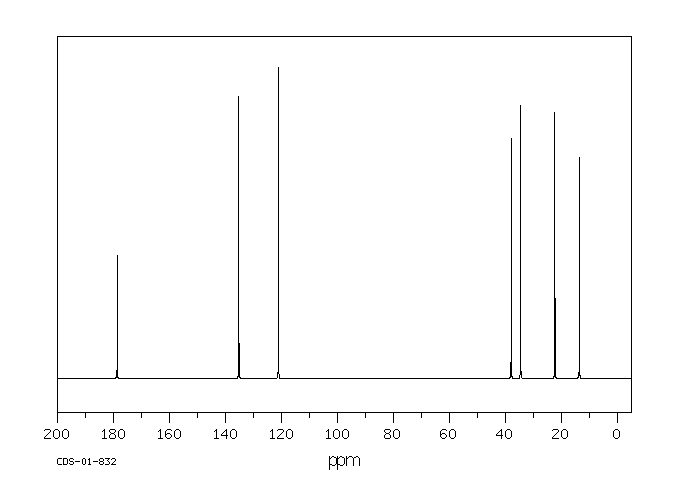
碳谱13CNMR
-
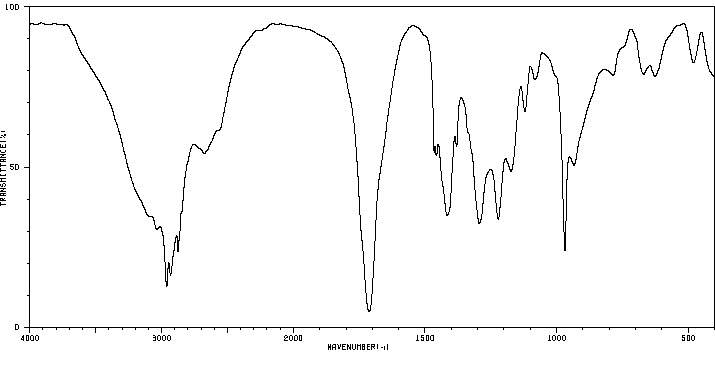
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯