异戊酰紫草素 | 52387-14-1
中文名称
异戊酰紫草素
中文别名
5,8-二羟基-2-(1-异氧戊酰-4-甲基-3-戊烯基)-1,4-萘二酮
英文名称
isovalerylshikonin
英文别名
(R)-1-(5,8-Dihydroxy-1,4-dioxo-1,4-dihydro-2-naphthyl)-4-methyl-3-pentenyl isovalerate;[(1R)-1-(5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl] 3-methylbutanoate
CAS
52387-14-1
化学式
C21H24O6
mdl
——
分子量
372.418
InChiKey
UTOUNDHZJFIVPK-QGZVFWFLSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:107 °C
-
沸点:570.1±50.0 °C(Predicted)
-
密度:1.246±0.06 g/cm3(Predicted)
-
LogP:4.073 (est)
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,目前没有已知危险反应。
计算性质
-
辛醇/水分配系数(LogP):4.8
-
重原子数:27
-
可旋转键数:7
-
环数:2.0
-
sp3杂化的碳原子比例:0.38
-
拓扑面积:101
-
氢给体数:2
-
氢受体数:6
安全信息
-
海关编码:2915900090
-
储存条件:请将贮藏器保持密封,并存放在阴凉、干燥处。同时,确保工作环境有足够的通风或排气设施。
SDS
上下游信息
反应信息
-
作为产物:描述:参考文献:名称:Human ACAT inhibitory effects of shikonin derivatives from Lithospermum erythrorhizon摘要:Three naphthoquinones were isolated by bioassay-guided fractionation from the CHCl3 extracts of roots of Lithospermum erythrorhizon. They were identified as acetylshikonin (1), isobutyrylshikonin (2), and (3-hydroxyisovalerylshikonin (3) on the basis of their spectroscopic analyses. The compounds 1-3 were tested for their inhibitory activities against human ACAT-1 (hACAT-1) or human ACAT-2 (hACAT-2). Compound 2 preferentially inhibited hACAT-2 (IC50 = 57.5 wM) than hACAT-1 (32% at 120 mu M), whereas compounds 1 and 3 showed weak inhibitory activities in both hACAT-1 and -2. To develop more potent hACAT inhibitor, shikonin derivatives (5-11) were synthesized by semi-synthesis of shikonin (4), which was prepared by hydrolysis of 1-3. Among them, compounds 5 and 7 exhibited the strong inhibitory activities against hACAT-1 and -2. Furthermore, we demonstrated that compound 7 behaved as a potent ACAT inhibitor in not only in vitro assay system but also cell-based assay system. (c) 2006 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmcl.2006.11.024
文献信息
-
Synthesis and Pharmacological In Vitro Investigations of Novel Shikonin Derivatives with a Special Focus on Cyclopropane Bearing Derivatives作者:Nadine Kretschmer、Antje Hufner、Christin Durchschein、Katrin Popodi、Beate Rinner、Birgit Lohberger、Rudolf BauerDOI:10.3390/ijms22052774日期:——activity by synthesis. Therefore, we prepared 31, mainly novel shikonin derivatives and screened them in different melanoma cell lines (WM9, WM164, and MUG-Mel2 cells) using the XTT viability assay. We identified (R)-1-(1,4-dihydro-5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl 2-cyclopropyl-2-oxoacetate as a novel derivative with even higher activity. Furthermore, pharmacological investigations黑色素瘤是最致命的皮肤癌形式,约占所有皮肤癌死亡人数的四分之三。尤其是在晚期,其治疗具有挑战性,并且存活率非常低。在先前的研究中,我们显示了Onosma paniculata根的成分以及活性最高的成分的合成衍生物在转移性黑色素瘤细胞系中显示出可喜的结果。在当前的研究中,我们解决了我们是否可以通过合成生成具有优化活性的其他衍生物的问题。因此,我们准备了31种主要是新型的紫草素衍生物,并使用XTT活力测定法在不同的黑色素瘤细胞系(WM9,WM164和MUG-Mel2细胞)中对其进行了筛选。我们确定([R)-1-(1,4-二氢-5,8-二羟基-1,4-二氧杂萘-2-基)-4-甲基戊-3-烯基2-环丙基-2-氧乙酸酯是具有更高活性的新型衍生物。此外,包括ApoToxGlo TM Triplex分析,LDH分析和细胞周期测量在内的药理研究表明,该化合物可诱导凋亡,并减少G1期的细胞,同时增加G2 /
-
Method of producing ginsenoside 20 (R)-Rh2 and composition of matter thereof申请人:Young Jeffrey公开号:US20050287230A1公开(公告)日:2005-12-29The present invention is a method of producing ginsenoside comprising the steps of: (a) producing a predetermined quantity of ginseng dialcohol from an extraction process of ginseng dialcohol; (b) reacting the ginseng dialcohol with a predetermined quantity of acetobromo-α-D-glucose under a predetermined condition; and (c) obtaining a predetermined quantity of ginsenoside Rh 2 . The present invention also provides a composition for enhancing the immune system of a living object comprising a quantity of ginsenoside, a quantity of ursolic acid and a quantity of shikonin. The composition may also comprise a quantity of panaxans, astragalan, and/or lentinan. The ginsenoside 20(R)-Rh 2 , ursolic acid, shikonin, panaxans, astragalan and lentinan have an effective range between 0.001 mg/kg and 0.1 mg/kg, 0.5 mg/kg and 10 mg/kg, 0.5 mg/kg and 10 mg/kg, 0.1 mg/kg and 1 mg/kg, 0.1 mg/kg and 1 mg/kg, and 0.1 mg/kg and 1 mg/kg respectively.本发明涉及一种生产人参皂苷的方法,包括以下步骤:(a)从人参二醇的提取过程中生产出预定量的人参二醇;(b)在预定条件下将人参二醇与预定量的乙酰溴-α-D-葡萄糖反应;(c)获得预定量的人参皂苷Rh2。本发明还提供了一种增强生物体免疫系统的组合物,包括一定量的人参皂苷、一定量的熊果酸和一定量的赤芍。该组合物还可以包括一定量的人参皂苷、黄芪苷和/或香菇多糖。人参皂苷20(R)-Rh2、熊果酸、赤芍、人参皂苷、黄芪苷和香菇多糖在0.001 mg/kg至0.1 mg/kg、0.5 mg/kg至10 mg/kg、0.5 mg/kg至10 mg/kg、0.1 mg/kg至1 mg/kg、0.1 mg/kg至1 mg/kg和0.1 mg/kg至1 mg/kg之间具有有效范围。
-
PROCESS FOR PRODUCING BLACK COLORANT, COSMETIC CONTAINING SAID COLORANT, AND APPLICATION THEREOF申请人:IHARA CHEMICAL INDUSTRY CO., LTD.公开号:EP0500942A1公开(公告)日:1992-09-02A process for producing a black colorant which comprises treating finely powdered amino sugar, protein or polyamide with a naphthoquinone derivative of general formula (1); a cosmetic containing a black colorant thus produced; and a process for coloring a base material with this colorant. In the said formula, R₁ represents hydrogen, hydroxy, halogen, α or β ; R₂ represents hydrogen or hydroxy; R₃ represents alkyl, alkenyl, or hydroxyalkyl: and n is 1 or 2.
-
ANTIPROTOZOAL AGENT申请人:Japan Health Sciences Foundation公开号:EP1779892A1公开(公告)日:2007-05-02The present invention provides an antiprotozoal agent, which is particularly preferred as an antileishmanial agent. Specifically, the present invention is directed to an antiprotozoal agent comprising, as an active ingredient, a compound of Formula (I): wherein R is -H or acyl, a salt thereof, or solvate thereof.本发明提供了一种抗原虫制剂,特别优选作为抗利什曼病制剂。具体地说,本发明涉及一种抗原虫制剂,其活性成分包括式(I)化合物: 其中 R 是-H 或酰基、 其盐或其溶液。
-
EXTERNAL PREPARATION申请人:Toyo Shinyaku Co., Ltd.公开号:EP1813250A1公开(公告)日:2007-08-01The present invention provides an external preparation that contains processed pueraria flower. When the external preparation of the present invention is applied to skin or mucous membranes, then skin quality improving effects (skin beautifying effects) such as preventing chapped skin or preventing wrinkles, the effect of strengthening mucous membrane elasticity, and the effect of preventing gingivitis and periodontal disease, for example, are obtained. The external preparation of the present invention can develop new use of the pueraria flower.本发明提供了一种含有加工过的葛根花的外用制剂。将本发明的外用制剂涂抹在皮肤或粘膜上,可获得改善皮肤质量的效果(美肤效果),例如防止皮肤干裂或防止皱纹、增强粘膜弹性的效果,以及预防牙龈炎和牙周病的效果。本发明的外用制剂可以开发葛根花的新用途。
表征谱图
-
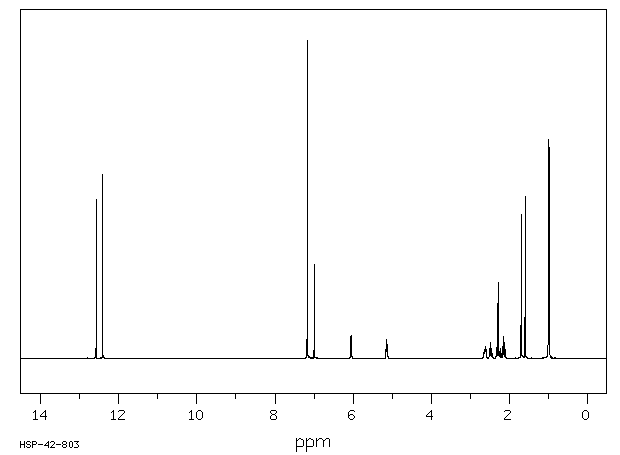
氢谱1HNMR
-
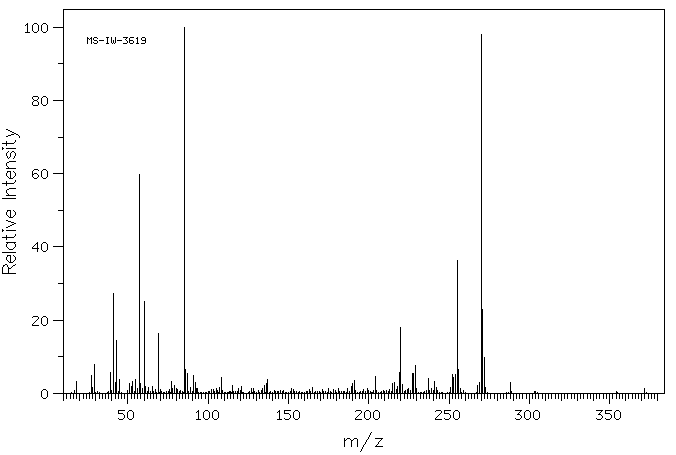
质谱MS
-
碳谱13CNMR
-
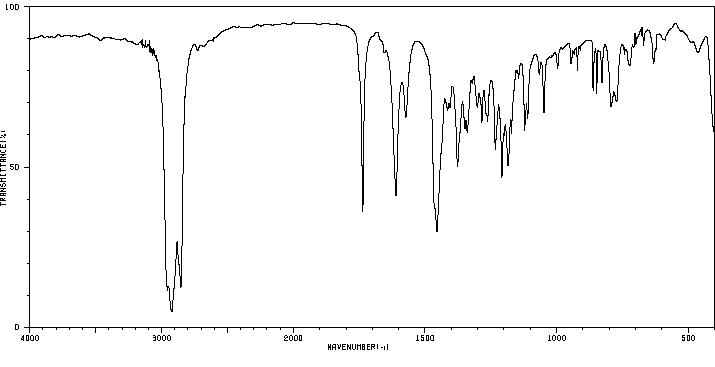
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮