1,2-丁二硫醇 | 16128-68-0
中文名称
1,2-丁二硫醇
中文别名
1,2-二巯基丁烷
英文名称
butane-1,2-dithiol
英文别名
1,2-butanedithiol;1.2-Dimercapto-butan;Butan-1,2-dithiol
CAS
16128-68-0
化学式
C4H10S2
mdl
MFCD00039649
分子量
122.255
InChiKey
LFTMJBWNOFFSRW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-53.9°C (estimate)
-
沸点:77°C/28mmHg(lit.)
-
密度:1.0390
-
LogP:2.19
-
物理描述:liquid with sulfury, roasted meat odour
-
溶解度:insoluble in water; miscible in fat
-
折光率:1.521-1.531
计算性质
-
辛醇/水分配系数(LogP):1.8
-
重原子数:6
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:2
-
氢给体数:2
-
氢受体数:2
安全信息
-
海关编码:2930909090
SDS
1,2-Butanedithiol Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 1,2-Butanedithiol
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS
Category 4
Flammable liquids
HEALTH HAZARDS
Category 2
Skin corrosion/irritation
Serious eye damage/eye irritation Category 2A
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Combustible liquid
Causes skin irritation
Causes serious eye irritation
Precautionary statements:
Keep away from flames and hot surfaces.
[Prevention]
Wash hands thoroughly after handling.
Wear protective gloves and eye/face protection.
[Response] IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
[Storage] Store in a well-ventilated place. Keep cool.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 1,2-Butanedithiol
Percent: >97.0%(GC)
16128-68-0
CAS Number:
Synonyms: 1,2-Dimercaptobutane
1,2-Butanedithiol
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Chemical Formula: C4H10S2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Get medical advice/attention if you feel unwell. Rinse mouth.
Ingestion:
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Dry chemical, foam, water spray, carbon dioxide.
Suitable extinguishing
media:
Unsuitable extinguishing Solid streams of water
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Prevention of secondary Remove all sources of ignition. Fire-extinguishing devices should be prepared in
case of a fire. Use spark-proof tools and explosion-proof equipment.
hazards:
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Keep away from flames and hot surfaces. Take
measures to prevent the build up of electrostatic charge. Use explosion-proof
equipment. Wash hands and face thoroughly after handling.
Use a closed system, ventilation.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool, dark and well-ventilated place.
Store under inert gas.
Store away from incompatible materials such as oxidizing agents.
Air-sensitive
Packaging material: Comply with laws.
1,2-Butanedithiol
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Clear
Form:
Colour: Colorless - Very pale yellow
No data available
Odour:
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: 77°C/3.7kPa
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: 1.04
Solubility(ies):
[Water] No data available
No data available
[Other solvents]
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Conditions to avoid: Open flame
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Sulfur oxides
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
1,2-Butanedithiol
Section 12. ECOLOGICAL INFORMATION
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Does not correspond to the classification standard of the United Nations
Hazards Class:
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 1,2-Butanedithiol
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS
Category 4
Flammable liquids
HEALTH HAZARDS
Category 2
Skin corrosion/irritation
Serious eye damage/eye irritation Category 2A
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Combustible liquid
Causes skin irritation
Causes serious eye irritation
Precautionary statements:
Keep away from flames and hot surfaces.
[Prevention]
Wash hands thoroughly after handling.
Wear protective gloves and eye/face protection.
[Response] IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
[Storage] Store in a well-ventilated place. Keep cool.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 1,2-Butanedithiol
Percent: >97.0%(GC)
16128-68-0
CAS Number:
Synonyms: 1,2-Dimercaptobutane
1,2-Butanedithiol
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Chemical Formula: C4H10S2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Get medical advice/attention if you feel unwell. Rinse mouth.
Ingestion:
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Dry chemical, foam, water spray, carbon dioxide.
Suitable extinguishing
media:
Unsuitable extinguishing Solid streams of water
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Prevention of secondary Remove all sources of ignition. Fire-extinguishing devices should be prepared in
case of a fire. Use spark-proof tools and explosion-proof equipment.
hazards:
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Keep away from flames and hot surfaces. Take
measures to prevent the build up of electrostatic charge. Use explosion-proof
equipment. Wash hands and face thoroughly after handling.
Use a closed system, ventilation.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool, dark and well-ventilated place.
Store under inert gas.
Store away from incompatible materials such as oxidizing agents.
Air-sensitive
Packaging material: Comply with laws.
1,2-Butanedithiol
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Clear
Form:
Colour: Colorless - Very pale yellow
No data available
Odour:
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: 77°C/3.7kPa
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: 1.04
Solubility(ies):
[Water] No data available
No data available
[Other solvents]
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Conditions to avoid: Open flame
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Sulfur oxides
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
1,2-Butanedithiol
Section 12. ECOLOGICAL INFORMATION
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Does not correspond to the classification standard of the United Nations
Hazards Class:
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
毒性
化学性质
用途
GRAS(FEMA)。
使用限量FEMA(mg/kg):在焙烤制品、肉制品、汤品、小吃食品、调味汁和果仁制品中,1,2-丁二硫醇的总用量不得超过1.0 mg/kg(即每种食品中的用量均不超过0.2 mg/kg)。
食品添加剂最大允许使用量与最大允许残留量标准| 添加剂中文名称 | 允许使用该种添加剂的食品中文名称 | 添加剂功能 | 最大允许使用量(g/kg) | 最大允许残留量(g/kg) |
|---|---|---|---|---|
| 1,2-丁二硫醇 | 食品 | 食品用香料 | 用于配制香精的各香料成分不得超过在GB 2760中的最大允许使用量和最大允许残留量 | - |
反应信息
-
作为反应物:参考文献:名称:Substituted dithiin tetroxide plant growth regulants摘要:植物生长的调节,包括除草剂、落叶剂或干燥剂效应,可以通过某些2,3-二氢-1,4-二硫杂环己烷-1,1,4,4-四氧化物来实现,其化学式为##STR1##其中R的值可以是氢、烷基等不同的取值。其中某些化合物,如2,3-二氢-5,6-二甲基-1,4-二硫杂环己烷-1,1,4,4-四氧化物是新的化学物质,可用作前和后出苗除草剂,并用于收获辅助程序,如对棉花和土豆等不同作物的落叶/干燥。公开号:US04276422A1
-
作为产物:描述:参考文献:名称:Simpson, Canadian Journal of Research, Section B: Chemical Sciences, 1947, vol. 25, p. 25摘要:DOI:
文献信息
-
A multinuclear (1H, 13C, 113Cd) nuclear magnetic resonance and magnetic circular dichroism spectroscopic study of thiolate complexes of cadmium作者:Geetha K. Carson、Philip A.W. Dean、Martin J. StillmanDOI:10.1016/s0020-1693(00)88548-5日期:1981.1chemical shifts are given for the complexes formed by 31 different thiolates individually in water at ambient probe temperature, and slow-exchange 1H and/or 13C and/or 113Cd NMR spectra are reported for several typical complexes at reduced temperature in CD3OD. In aqueous solution at ambient probe temperature, ligands with vicinal thiolate groups and −SCH2CHRS− (R = H, Me, Et, CH2OH, CH2SO−3 or CH2S−)) form1H,13C和113Cd NMR的适当组合已用于研究ca。在过量的硫醇盐存在下,由相应的难溶性硫氰酸镉形成的0.05M溶液中,富含113Cd的大量硫氰酸镧盐酸盐。此外,已记录了MCD光谱的稀度。10-5M,一组代表性的硫醇盐络合物的溶液。对于在环境探针温度下由31种不同的硫醇盐在水中分别形成的配合物,给出了镉113的化学位移,并且在CD3OD中报道了几种典型的配合物在降低的温度下的慢速交换1H和/或13C和/或113Cd NMR光谱。在环境探针温度下的水溶液中,具有邻位硫醇盐基团和-SCH2CHRS-(R = H,Me,Et,CH2OH, O-3或CH2S-)的配体形成具有δCd⪖778 ppm的双(螯合物)络合物,镉的局部微观对称性接近四面体。具体而言,在[Cd(SR)2.5] 0.5n-n(R = Me,Et或Pr)络合物在250 nm区域的S→Cd电荷转移带下表现出良好分辨的对称A项。二硫醇盐-S(CH2nS-(n
-
Reactions of Dithiolate Ligands in Mononuclear Complexes of Rhenium(V)作者:J. A. Kanney、B. C. Noll、M. Rakowski DuBoisDOI:10.1021/ja020125z日期:2002.8.1The thermal reactions of the Re(V) dithiolate complex Cp'ReCl2(SCH2CH2S), 1 (where Cp' = EtMe4C5), and related derivatives have been studied. When 1 is heated in toluene in a sealed evacuated tube at 100 degrees C, a dehydrogenation reaction occurs to form a new rhenium complex with a dithiolene ligand, Cp'ReCl2(SCHCHS), 6, in ca. 40% yield. The structure of 6 has been confirmed by an X-ray diffraction已经研究了 Re(V) 二硫醇复合物 Cp'ReCl2(SCH2CH2S), 1(其中 Cp' = EtMe4C5)和相关衍生物的热反应。当 1 在密封的真空管中在 100 摄氏度的甲苯中加热时,会发生脱氢反应,与二硫烯配体 Cp'ReCl2(SCHCHS), 6 形成新的铼配合物。40% 的收率。X 射线衍射研究证实了 6 的结构。在所研究的热条件下,1 也经历了烯烃挤出反应。在产物的核磁共振谱中检测到游离乙烯,同时也形成了不溶性铼产物。当 1 在温和条件下与过量的乙烯反应时,会形成新的有机产物 1,4-二噻烷。还发现配合物 1 在温和条件下与氧化剂(如 O2 和 S8)反应形成脱氢产物 6。
-
Herbicidal method employing substituted dithin tetroxides申请人:Uniroyal Inc.公开号:US03997323A1公开(公告)日:1976-12-14Regulation of the growth of plant life, including herbicidal, defoliant or desiccant effects, may be accomplished with certain 2,3-dihydro-1,4-dithiin 1,1,4,4-tetroxides of the formula ##STR1## wherein the R's have various values such as hydrogen, alkyl, etc. Certain of the compounds, such as 2,3-dihydro-5,6-dimethyl-1,4-dithiin 1,1,4,4-tetroxide, are new chemicals, and are useful as pre-and post-emergent herbicides, and in harvest aid procedures, such as defoliation/desiccation of various crops, including cotton and potatoes.
-
Method of making certain 2,3-dihydro-1,4-dithiins申请人:Uniroyal Ltd.公开号:US04319033A1公开(公告)日:1982-03-09A method of making certain 2,3-dihydro-1,4-dithiins of the following formula by the action of alpha-hydroxyketones (acyloins) of 1,2-dithiols: ##STR1## wherein R.sup.1 and R.sup.2 are hydrogen or alkyl having 1 to 6 carbon atoms or are joined together to form a ring with 3 or 4 methylene groups, and R.sup.3 and R.sup.4 are hydrogen or the same or different alkyl groups having 1 to 10 carbon atoms, which alkyl groups may be substituted with lower alkoxy groups.
-
Selective Covalent Targeting of Pyruvate Kinase M2 Using Arsenous Warheads作者:Jingyao Wang、Shaoqing Zhou、Yan Cheng、Lin Cheng、Ying Qin、Zhenfeng Zhang、Aiwei Bi、Huaijiang Xiang、Xinheng He、Xiaoxu Tian、Wenbin Liu、Jian Zhang、Chao Peng、Zhengjiang Zhu、Min Huang、Ying Li、Guanglei Zhuang、Li TanDOI:10.1021/acs.jmedchem.2c01563日期:2023.2.23in covalent targeted inhibitors in drug discovery against previously “undruggable” sites and targets. These molecules typically feature an electrophilic warhead that reacts with nucleophilic groups of protein residues, most notably the thiol group of cysteines. One main challenge in the field is to develop versatile utilizable warheads. Here, we characterize the unique features of novel arsenous warheads在针对以前“不可药化”的位点和靶标的药物发现中,人们对共价靶向抑制剂的兴趣越来越大。这些分子通常具有与蛋白质残基的亲核基团反应的亲电子弹头,最显着的是半胱氨酸的硫醇基团。该领域的一项主要挑战是开发多功能的可利用弹头。在这里,我们描述了新型砷弹头以可逆方式与硫醇物种反应的独特特征,并进一步证明有机砷探针可以通过开发选择性和有效的丙酮酸激酶 M2 (PKM2) 抑制剂来化学调节特定分子靶标。我们表明化合物24是 PKM2 及其口服生物可利用前药25的共价变构抑制剂在体外和体内有效抑制 PKM2 依赖性肿瘤生长。我们的结果介绍了25及其衍生物作为有用的药理学工具,并提供了使用砷弹头靶向蛋白质半胱氨酸组的一般路线图。
表征谱图
-
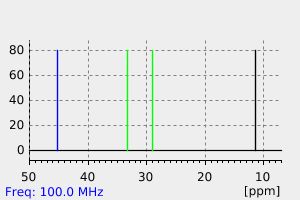
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
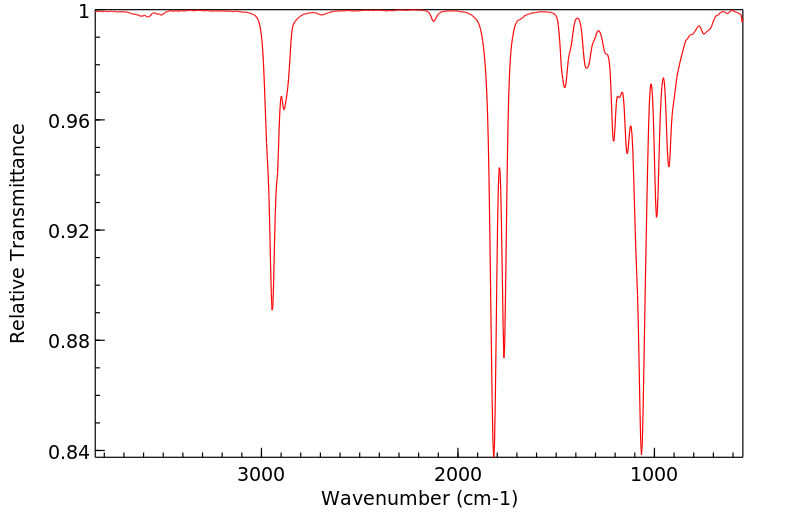
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
铜,丙烷-2-硫醇
铅,丙烷-1-硫醇
苏-(2R,4R)-戊二硫醇
羟基-乙醛
硫甘油
癸烷-2-硫醇
甲硫醇铅
甲硫醇钠
甲硫醇-d4
甲硫醇-S-d
甲硫醇
甲三硫醇
环辛硫醇
环戊硫醇
环戊基甲硫醇
环庚烷-1,1-二硫醇
环己硫醇
环己烷-1,1-二硫醇
环己基甲硫醇
环十二烷硫醇
环丙硫醇
环丙基甲硫醇
环丁硫醇
油烯基硫醇
氟甲硫醇
氘代甲硫醇-D3
正十四烷基硫醇
末端脱氧核苷酸转移酶
戊赤藓四硫醇
戊烷-3-硫醇
异戊硫醇
异戊烯基硫醇
异丙硫醇
异丁硫醇
庚-3-烯-4-硫醇
己-2-烯-1-硫醇
巯基甲烷-13C
巯基乙醛
巯基乙胺氢溴酸盐
巯基乙胺
巯基-十一胺盐酸盐
壬烷-2-硫醇
吡啶,2-(戊基硫代)-,1-氧化
叔壬基硫醇
叔十六硫醇
叔十二烷硫醇
叔丁基硫醇
反式-2-丁烯-1-硫醇
双(巯基环戊烷)四氯化钛
半胱胺盐酸盐