1,3-二氯-4-三甲基苯 | 13014-18-1
中文名称
1,3-二氯-4-三甲基苯
中文别名
2,4-二氯三氯甲苯;2,4-二氯三氯苄;α,α,α,2,4-五氯甲苯
英文名称
2,4-dichlorobenzotrichloride
英文别名
2,4-dichloro-1-(trichloromethyl)benzene
CAS
13014-18-1
化学式
C7H3Cl5
mdl
MFCD00018818
分子量
264.366
InChiKey
KZSNBJMYJWDVTK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:49°C
-
沸点:288°C
-
密度:1.5935 (estimate)
-
闪点:>112℃
-
溶解度:氯仿(微溶)、甲醇(微溶)
-
LogP:5.18 at 25℃
-
物理描述:2,4,alpha,alpha,alpha-pentachlorotoluene is a white crystalline powder. (NTP, 1992)
-
保留指数:1557.6
计算性质
-
辛醇/水分配系数(LogP):5.5
-
重原子数:12
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
安全说明:S26,S36/37/39
-
危险品运输编号:3261
-
海关编码:2903999090
-
危险类别码:R34
-
RTECS号:CZ5475020
-
储存条件:| 室温 |
SDS
Section I.Chemical Product and Company Identification
Chemical Name 2,4-Dichlorobenzotrichloride
Portland OR
Synonym Benzene, 2,4-Dichloro-1-(trichloromethyl)- ( 9 CI)
Chemical Formula CCl3C6H3Cl2
CAS Number 13014-18-1
Section II. Composition and Information on Ingredients
Chemical Name CAS Number Percent (%) TLV/PEL Toxicology Data
2,4-Dichlorobenzotrichloride 13014-18-1 Min. 99.0 Not available. Rat LD50 (oral) 3160mg/kg
Rabbit LD (dermal) >10gm/kg
(GC)
Section III. Hazards Identification
Acute Health Effects Corrosive to skin, eyes, and respiratory system. Liquid or spray mist may produce tissue damage, particularly in mucous
membranes of the eyes, mouth and respiratory tract. Skin contact may produce burns. Eye contact can result in corneal
damage or blindness. Inhalation of the spray mist may produce severe irritation of respiratory tract, characterized by
coughing, choking, or shortness of breath. Corrosive materials may cause serious injury if ingested. Follow safe
industrial hygiene practices and always wear proper protective equipment when handling this compound.
Chronic Health Effects CARCINOGENIC EFFECTS : Not available.
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
Repeated exposure of the eyes to a low level of dust can produce eye irritation. Repeated skin exposure can produce
local skin destruction, or dermatitis. Repeated inhalation of dust can produce varying degree of respiratory irritation or
lung damage.
Section IV. First Aid Measures
Eye Contact Check for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15
minutes. Get medical attention.
Skin Contact In case of contact, immediately flush skin with plenty of water. Remove contaminated clothing and shoes. Wash clothing
before reuse. Thoroughly clean shoes before reuse. Get medical attention.
Inhalation If the victim is not breathing, perform mouth-to-mouth resuscitation. Loosen tight clothing such as a collar, tie, belt or
waistband. If breathing is difficult, oxygen can be administered. Seek medical attention if respiration problems do not
improve.
Ingestion DO NOT INDUCE VOMITING. Loosen tight clothing such as a collar, tie, belt or waistband. If the victim is not breathing,
perform mouth-to-mouth resuscitation. Examine the lips and mouth to ascertain whether the tissues are damaged, a
possible indication that the toxic material was ingested; the absence of such signs, however, is not conclusive.
Section V. Fire and Explosion Data
Auto-Ignition Not available.
Flammability May be combustible at high temperature.
Flash Points Flammable Limits Not available.
Not available.
Combustion Products These products are toxic carbon oxides (CO, CO 2), halogenated compounds.
WARNING: Highly toxic HCl gas is produced during combustion.
Fire Hazards
Not available.
Explosion Hazards Risks of explosion of the product in presence of mechanical impact: Not available.
Risks of explosion of the product in presence of static discharge: Not available.
Fire Fighting Media
SMALL FIRE: Use DRY chemical powder.
and Instructions LARGE FIRE: Use water spray, fog or foam. DO NOT use water jet.
Consult with local fire authorities before attempting large scale fire-fighting operations.
Continued on Next Page
2,4-Dichlorobenzotrichloride
Section VI. Accidental Release Measures
Spill Cleanup Corrosive solid.
Instructions Stop leak if without risk. DO NOT get water inside container. DO NOT touch spilled material. Use water spray to reduce
vapors. Prevent entry into sewers, basements or confined areas; dike if needed. Eliminate all sources of ignition.
Consult federal, state, and/or local authorities for assistance on disposal.
Section VII. Handling and Storage
Handling and Storage CORROSIVE. Keep container dry. Keep away from heat. Mechanical exhaust required. When not in use, tightly seal the
container and store in a dry, cool place. Avoid excessive heat and light. Do not breathe dust. Never add water to this
Information
product. Wear suitable protective clothing. If you feel unwell, seek medical attention and show the label when possible.
Treat symptomatically and supportively.
Always store away from incompatible compounds such as oxidizing agents.
Section VIII. Exposure Controls/Personal Protection
Engineering Controls Use process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below
recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to
airborne contaminants below the exposure limit.
Personal Protection Face shield. Lab coat. Dust respirator. Boots. Gloves. A MSHA/NIOSH approved respirator must be used to avoid
inhalation of the product. Suggested protective clothing might not be sufficient; consult a specialist BEFORE handling
this product.
Exposure Limits Not available.
Section IX. Physical and Chemical Properties
Physical state @ 20°C Solid. Solubility
Not available.
Not available.
Specific Gravity
Molecular Weight 264.36 Partition Coefficient
Not available.
Boiling Point Not available. Vapor Pressure Not applicable.
Melting Point 49°C (120.2°F) Vapor Density Not available.
Not available. Not available.
Refractive Index Volatility
Critical Temperature Not available. Odor Not available.
Viscosity Not available. Taste Not available.
Section X. Stability and Reactivity Data
Stability
This material is stable if stored under proper conditions. (See Section VII for instructions)
Conditions of Instability
Avoid excessive heat and light.
Incompatibilities
Reactive with oxidizing agents.
Section XI. Toxicological Information
RTECS Number CZ5475020
Routes of Exposure Eye Contact. Ingestion. inhalation. Skin contact.
Rat LD50 (oral) 3160mg/kg
Toxicity Data
Rabbit LD (dermal) >10gm/kg
Chronic Toxic Effects CARCINOGENIC EFFECTS : Not available.
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
Repeated exposure of the eyes to a low level of dust can produce eye irritation. Repeated skin exposure can produce
local skin destruction, or dermatitis. Repeated inhalation of dust can produce varying degree of respiratory irritation or
lung damage.
Acute Toxic Effects Corrosive to skin, eyes, and respiratory system. Liquid or spray mist may produce tissue damage, particularly in mucous
membranes of the eyes, mouth and respiratory tract. Skin contact may produce burns. Eye contact can result in corneal
damage or blindness. Inhalation of the spray mist may produce severe irritation of respiratory tract, characterized by
coughing, choking, or shortness of breath. Corrosive materials may cause serious injury if ingested. Follow safe
industrial hygiene practices and always wear proper protective equipment when handling this compound.
Continued on Next Page
2,4-Dichlorobenzotrichloride
Section XII. Ecological Information
Ecotoxicity Not available.
Environmental Fate Not available.
Section XIII. Disposal Considerations
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material with a
Waste Disposal
combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system. Observe all
federal, state and local regulations when disposing of the substance.
Section XIV. Transport Information
DOT Classification Class 8: Corrosive material
PIN Number
Proper Shipping Name
Corrosive solid, n.o.s.
Packing Group (PG) III
DOT Pictograms
Section XV. Other Regulatory Information and Pictograms
TSCA Chemical Inventory This compound is ON the EPA Toxic Substances Control Act (TSCA) inventory list.
(EPA)
WHMIS Classification
CLASS E: Corrosive solid.
On NDSL.
(Canada)
EINECS Number (EEC) 235-868-1
EEC Risk Statements
R36/37/38- Irritating to eyes, respiratory system and skin.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
用途:医药、化工。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,4-二氯甲苯 2,4-dichlorotoluene 95-73-8 C7H6Cl2 161.031 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2,4-二氯苯甲酰氯 2,4-dichlorobenzoyl chloride 89-75-8 C7H3Cl3O 209.459
反应信息
-
作为反应物:描述:1,3-二氯-4-三甲基苯 在 iron(III) chloride 作用下, 以 水 为溶剂, 反应 2.0h, 以93.05%的产率得到2,4-二氯苯甲酰氯参考文献:名称:一种2,4-二氯苯甲酰氯的制备方法摘要:本发明提供一种2,4‑二氯苯甲酰氯的制备方法,涉及精细化工有机合成技术领域,包括以下步骤:(1)在催化剂偶氮二异丁腈作用下,将2,4‑二氯甲苯和氯气发生氯代反应,制备2,4‑二氯三氯甲苯粗品;(2)将步骤(1)制备的2,4‑二氯三氯甲苯粗品和水发生水解反应的;(3)将步骤(2)的水解产物通过减压精馏,收集区域的馏分制备目标产物2,4‑二氯苯甲酰氯,本发明所述工艺原材料易得,价格便宜,操作简单,能耗低,产品质量高,生产成本低,三废少,产品总收率在90%‑95%之间,纯度99%以上,适合工业化生产,具有很好的应用前景。公开号:CN109678698B
-
作为产物:描述:参考文献:名称:一种二氯三氟甲苯的高效氯化工艺摘要:本发明公开了一种二氯三氟甲苯的高效氯化工艺,属于有机合成领域。该法以二氯甲苯为原料,加入氯化亚铜与铜粉的混合物催化剂,经过氯化、氟化二步反应得到目标化合物,反应过程中应用的原料方便购得,生产成本低,后处理简单,反应条件温和,具有良好的经济效益和社会效益。公开号:CN110483237A
文献信息
-
One-Pot Synthesis of Tertiary Amides from Organic Trichlorides through Oxygen Atom Incorporation from Air by Convergent Paired Electrolysis作者:Zhongli Luo、Kenji Imamura、Yoshihito Shiota、Kazunari Yoshizawa、Yoshio Hisaeda、Hisashi ShimakoshiDOI:10.1021/acs.joc.1c00161日期:2021.4.16A convergent paired electrolysis catalyzed by a B12 complex for the one-pot synthesis of a tertiary amide from organic trichlorides (R-CCl3) has been developed. Various readily available organic trichlorides, such as benzotrichloride and its derivatives, chloroform, dichlorodiphenyltrichloroethane (DDT), trichloro-2,2,2-trifluoroethane (CFC-113a), and trichloroacetonitrile (CNCCl3), were converted
-
Visible light-driven photocatalytic duet reaction catalyzed by the B12-rhodium-titanium oxide hybrid catalyst作者:Keita Shichijo、Mamoru Fujitsuka、Yoshio Hisaeda、Hisashi ShimakoshiDOI:10.1016/j.jorganchem.2019.121058日期:2020.2imply that electron transfer from the titanium oxide to Co(III) center of the B12 complex occurred by the visible light irradiation. Benzotrichloride was converted to N,N-diethylbenzamide by the visible light irradiation catalyzed by the hybrid catalyst in air at room temperature. Both the conduction band electron and valence band hole of the catalyst were utilized for the reaction to form the amide合成了由B 12配合物和铑离子(Rh 3+)改性的氧化钛组成的杂化催化剂,用于可见光驱动的B 12激发的催化反应。杂化催化剂在氧化钛表面上含有4.93×10 -6 molg -1的B 12络合物和5.43×10 -5 molg -1的Rh(III)离子。在作为牺牲试剂的三乙胺(Et 3 N)存在下,杂化催化剂的可见光照射(λ≥420 nm)显示出在B 12的Co(I)状态下典型的390 nm吸收。通过漫反射紫外可见光谱分析监测该配合物,这意味着电子从二氧化钛转移到B 12配合物的Co(III)中心是通过可见光辐射发生的。在室温下,在空气中,由杂化催化剂催化的可见光辐射将三氯化苯转化为N,N-二乙基苯甲酰胺。催化剂的导带电子和价带孔均用于反应以形成酰胺产物。提出了二重反应的反应机理。
-
Visible Light‐Driven, One‐pot Amide Synthesis Catalyzed by the B<sub>12</sub>Model Complex under Aerobic Conditions作者:Hui Tian、Hisashi Shimakoshi、Toshikazu Ono、Yoshio HisaedaDOI:10.1002/cplu.201800522日期:2019.3was developed. It provides a convenient and efficient way to synthesize amides. Based on this method, trichlorinated organic compounds were converted into amides in the presence of an amine under aerobic conditions at room temperature in a one-pot procedure. Various trichlorinated organic compounds and an amine source, such as primary, secondary, and cyclic amines, have been evaluated for this transformation
-
一种2,4-二氯三氟甲苯的高效氯化工艺
-
Oxygen‐Controlled Catalysis by Vitamin B <sub>12</sub> ‐TiO <sub>2</sub> : Formation of Esters and Amides from Trichlorinated Organic Compounds by Photoirradiation作者:Hisashi Shimakoshi、Yoshio HisaedaDOI:10.1002/anie.201507782日期:2015.12.14An oxygen switch in catalysis of the cobalamin derivative (B12)‐TiO2 hybrid catalyst for the dechlorination of trichlorinated organic compounds has been developed. The covalently bound B12 on the TiO2 surface transformed trichlorinated organic compounds into an ester and amide by UV light irradiation under mild conditions (in air at room temperature), while dichlorostilbenes (E and Z forms) were formed
表征谱图
-
氢谱1HNMR
-
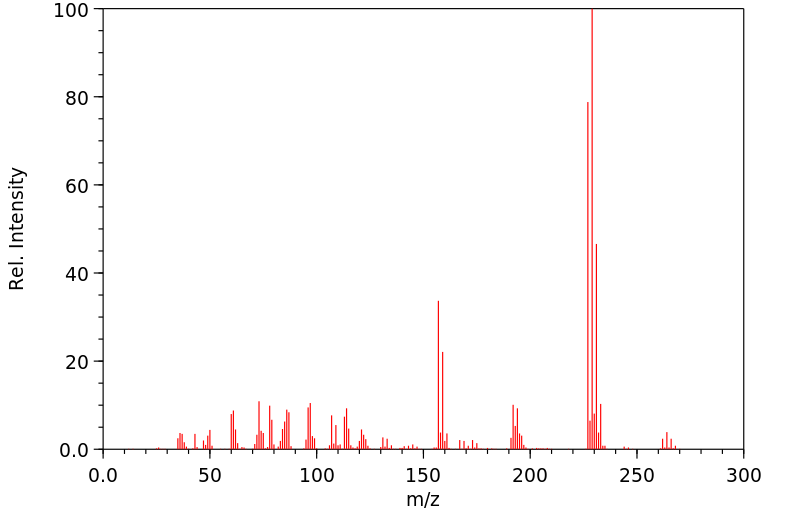
质谱MS
-
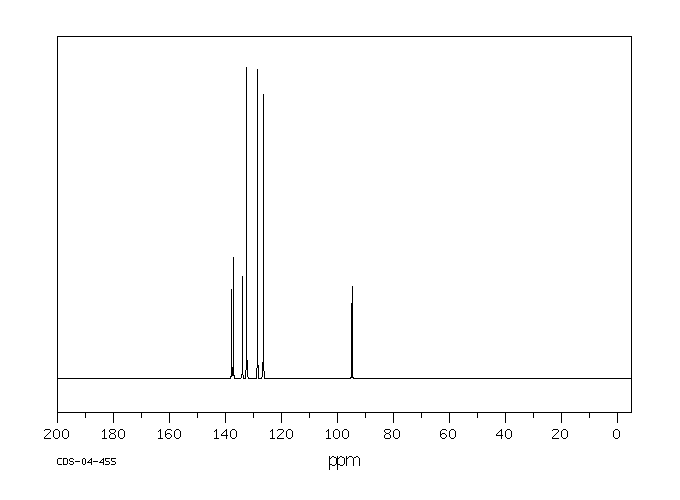
碳谱13CNMR
-
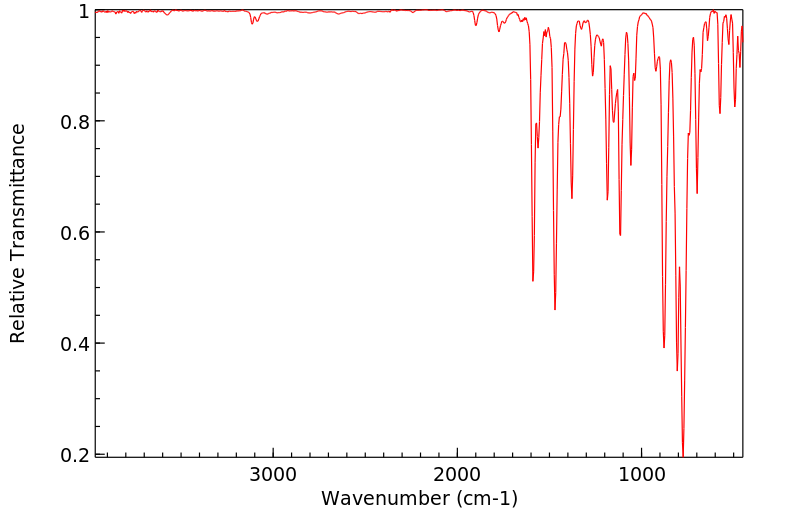
红外IR
-
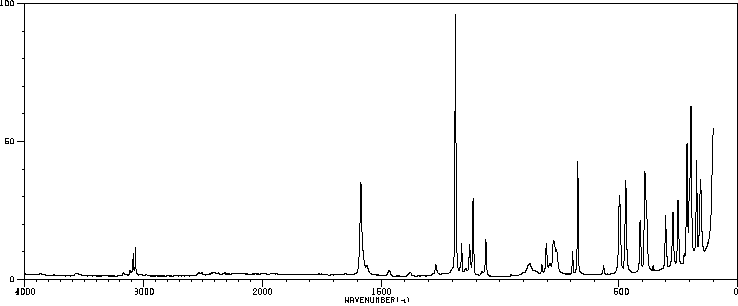
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫