1,4,4a,9alpha-四氢蒽醌 | 56136-14-2
中文名称
1,4,4a,9alpha-四氢蒽醌
中文别名
——
英文名称
1,4,4a,9a-tetrahydroanthraquinone
英文别名
1,4,4a,9a-Tetrahydro-anthrachinon;9,10-Anthracenedione, 1,4,4a,9a-tetrahydro-;1,4,4a,9a-tetrahydroanthracene-9,10-dione
CAS
56136-14-2
化学式
C14H12O2
mdl
MFCD00009678
分子量
212.248
InChiKey
XPCZSIPRUSOJFO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:99-101 °C(lit.)
-
沸点:382.5±42.0 °C(Predicted)
-
密度:1.216±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:16
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.285
-
拓扑面积:34.1
-
氢给体数:0
-
氢受体数:2
安全信息
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
SDS
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Diels; Alder, Justus Liebigs Annalen der Chemie, 1928, vol. 460, p. 98摘要:DOI:
-
作为产物:描述:1,4-萘醌 以80%的产率得到参考文献:名称:TANDON V. K.; SINGH R.; KHANNA J. M.; ANAND N., INDIAN J. CHEM., 1977, B15, NO 9, 839-842摘要:DOI:
文献信息
-
A Cheap, Catalytic, Scalable, and Environmentally Benign Method for Alkene Epoxidations作者:Benjamin S. Lane、Kevin BurgessDOI:10.1021/ja004000a日期:2001.3.1aliphatic alkenes can be selectively epoxidized in the presence of terminal alkenes. This experiment also implies that the allylic hydroxyl does not activate the terminal alkene via a directing effect. Entries 9-12 illustrate that epoxidations of arylsubstituted alkenes proceed smoothly; qualitatively, the rates of these reactions were observed to be appreciably faster than for aliphatic alkenes. The only烯烃环氧化的良性方法 Benjamin S. Lane 和 Kevin Burgess* 德克萨斯 A & M 大学化学系 PO Box 30012, College STAtion, Texas 77842-3012 收到 2000 年 11 月 17 日 本文报告了一种简单的方法,其中锰 (2+)盐,例如 MnSO4,使用 30% 的过氧化氢水溶液作为末端氧化剂催化烯烃的环氧化。通过将底物和催化剂溶解在 DMF 或叔丁醇中,然后缓慢加入 30% 过氧化氢和 0.2 M 碳酸氢钠缓冲液的混合物来进行反应。这种方法在成本、简单性和环境因素方面有几个理想的属性。该项目来自在筛选新的、手性的、1,4、7-三氮杂环壬烷 (TACN) 配合物作为潜在的不对称环氧化催化剂。在一个简单的平板装置 1 中的高通量筛选表明,没有任何有机配体的简单锰 (2+) 盐介导了环氧化,但仅在碳酸氢盐缓冲液中。在基于
-
Process for the preparation of mononitro-1,2,3,4-tetrahydroanthraquinones申请人:Produits Chimiques Ugine Kuhlmann公开号:US04283345A1公开(公告)日:1981-08-11A process is disclosed for the preparation of mononitro derivatives of 1,2,3,4-tetrahydro-anthraquinone from 1,4,4a,9a-tetrahydro-anthraquinone, in which a thermal pretreatment of the 1,4,4a,9a-tetrahydro-anthraquinone is carried out in the presence of a hydrogenation catalyst in an inert atmosphere and in the absence of oxidizing or reducing agents, the anthraquinone by-produced as well as the catalyst are separated and the mixture of 1,2,3,4-tetrahydro-9,10-anthracenediol and 1,2,3,4-tetrahydro-anthraquinone obtained is subjected to a nitration reaction.
-
New Catalytic Reactions in the Presence of Mo-V-Phosphoric Heteropoly Acid Solutions作者:E. G. Zhizhina、M. V. Simonova、V. V. Russkikh、K. I. MatveevDOI:10.1007/s11167-005-0386-9日期:2005.5The possibility of performing new catalytic reactions in the presence of solutions of H x+3PMo12−x . VxO40 Mo-V-phosphoric heteropoly acids was examined.研究了在 H x+3PMo12-x .VxO40 Mo-V-磷酸杂多酸溶液中进行新催化反应的可能性进行了研究。
-
Process for the preparation of 1,2,3,4-tetrahydro-9,10-anthracene-diol申请人:PCUK - Produits Chimiques Ugine Kuhlmann公开号:US04365100A1公开(公告)日:1982-12-21A process for the preparation of 1,2,3,4-tetrahydro-9,10-anthracene-diol, in which catalytic hydrogenation of 1,4,4a,9a-tetrahydro-anthraquinone is carried out in the liquid phase to give 1,2,3,4,4a,9a-hexahydro-9,10-anthracene-dione followed by isomerization of the latter in the presence of an acid to give 1,2,3,4-tetrahydro-9,10-anthracene-diol.
-
meso-Tetraphenylporphyrin with a pi-system extended by fusion with anthraquinone作者:Mikhail A. Filatov、Ernesta Heinrich、Katharina Landfester、Stanislav BaluschevDOI:10.1039/c5ob00884k日期:——
Fusion of a porphyrin with anthraquinone through the synthesis of a suitably substituted pyrrole that can be cyclotetramerized and the optical properties of the resulting molecular system are described.
表征谱图
-
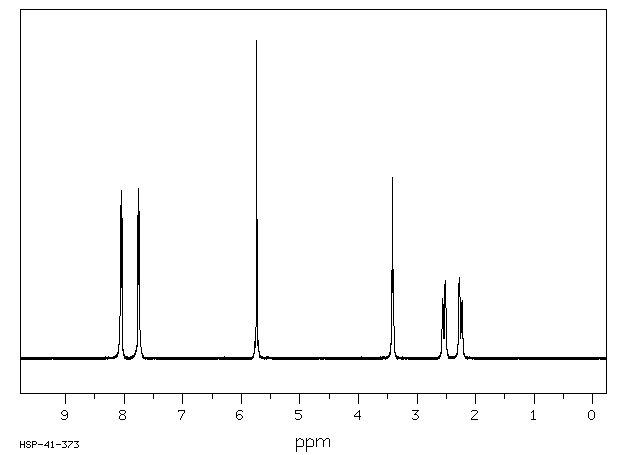
氢谱1HNMR
-
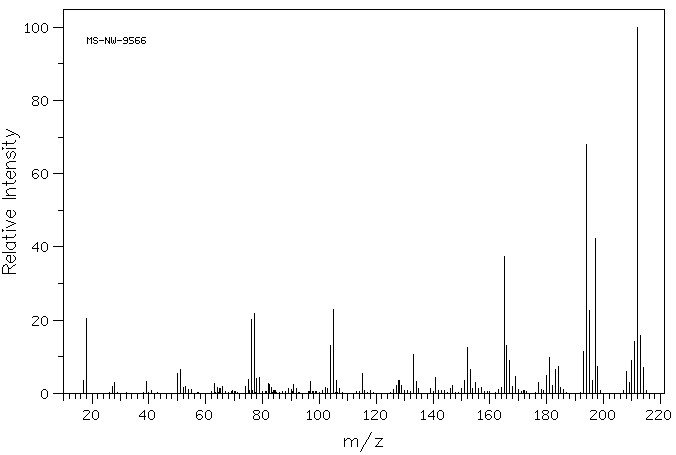
质谱MS
-
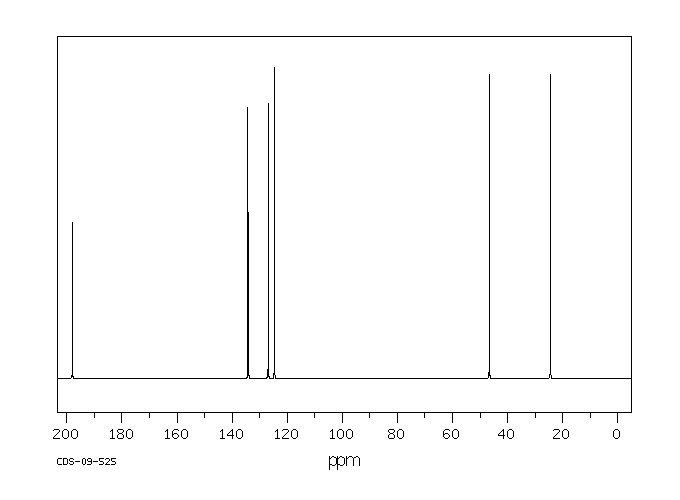
碳谱13CNMR
-
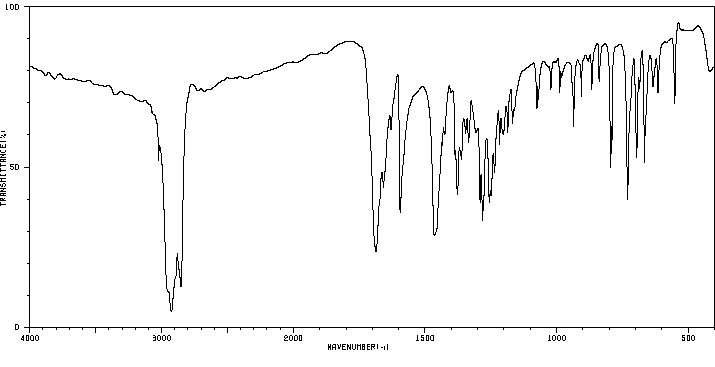
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
齐斯托醌
黄决明素
马普替林相关物质D
马普替林杂质E(N-甲基马普替林)
马普替林杂质D
马普替林D3
马普替林
颜料黄199
颜料黄147
颜料黄123
颜料黄108
颜料红89
颜料红85
颜料红251
颜料红177
颜料紫27
顺式-1-(9-蒽基)-2-硝基乙烯
阿美蒽醌
阳离子蓝FGL
阳离子蓝3RL
长蠕孢素
镁蒽四氢呋喃络合物
镁蒽
锈色洋地黄醌醇
锂钠2-[[4-[[3-[(4-氨基-9,10-二氧代-3-磺基-1-蒽基)氨基]-2,2-二甲基-丙基]氨基]-6-氯-1,3,5-三嗪-2-基]氨基]苯-1,4-二磺酸酯
锂胭脂红
链蠕孢素
铷离子载体I
铝洋红
铂(2+)二氯化1-({2-[(2-氨基乙基)氨基]乙基}氨基)蒽-9,10-二酮(1:1)
钾6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠alpha-(丙烯酰氨基)-[4-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]苯氧基]甲苯磺酸盐
钠[[3-[[4-(环己基氨基)-9,10-二氢-9,10-二氧代-1-蒽基]氨基]-1-氧代丙基]氨基]苯磺酸盐
钠[3-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]丁基]苯磺酸盐
钠6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠4-({4-[乙酰基(乙基)氨基]苯基}氨基)-1-氨基-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠2-[(4-氨基-9,10-二氧代-3-磺基-9,10-二氢-1-蒽基)氨基]-4-{[2-(磺基氧基)乙基]磺酰基}苯甲酸酯
钠1-氨基-9,10-二氢-4-[[4-(1,1-二甲基乙基)-2-甲基苯基]氨基]-9,10-二氧代蒽-2-磺酸盐
钠1-氨基-4-[(3-{[(4-甲基苯基)磺酰基]氨基}苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-[(3,4-二甲基苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-(1,3-苯并噻唑-2-基硫基)-9,10-二氧代蒽-2-磺酸盐
醌茜隐色体
醌茜素
酸性蓝P-RLS
酸性蓝41
酸性蓝27
酸性蓝127:1
酸性紫48
酸性紫43
酸性兰62